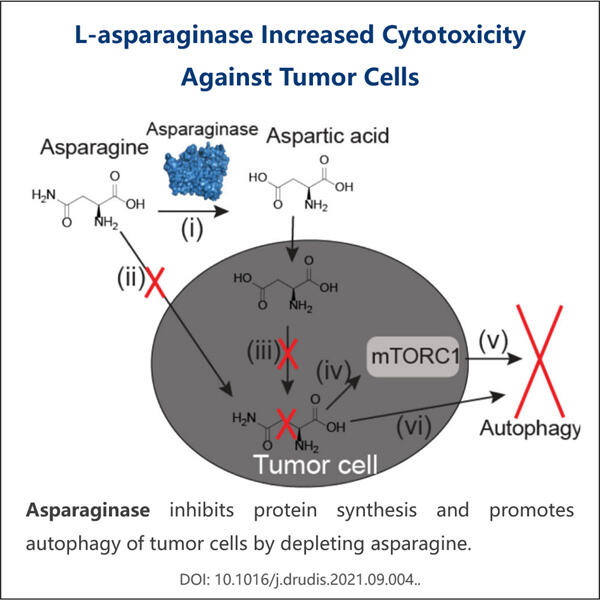
So, L-asparaginase is a high-impact medicine for patients with cancer. Cancer is a disease in which some of the body’s cells grow uncontrollably, and this can cause people to become very ill. L-asparaginase prevents further growth of such cancer cells. Cancer cells require a nutrient called asparagine to grow and thrive, and L-asparaginase prevents their body from producing it. This means that the medicine can prevent cancer from growing or spreading faster. Such medicine is made by companies like Yaohai which follow specific regulations to ensure the safety and efficacy of the medicine.
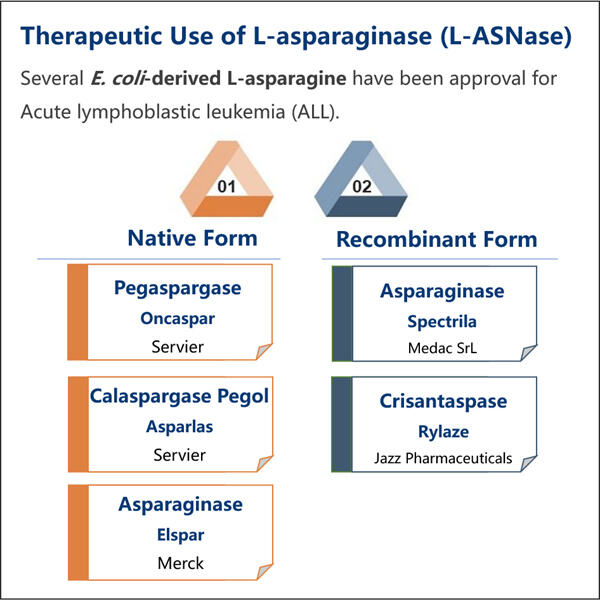
The very first step in the production of L-asparaginase is to understand what type they want to produce. L-asparaginase comes in two main types that doctors might use: one that is bacteria-based and one that is animal-based. Yaohai prefers to produce the type that comes from bacteria because it is easier to control and safer for people to use. After the bacteria grows in certain conditions, it is harvested and L-asparaginase is extracted from the rest of the bacteria. This process is lengthy, consisting of numerous stages in order to ensure that the end product is pure and safe for patients.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 НЕМАЄ
НЕМАЄ
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN