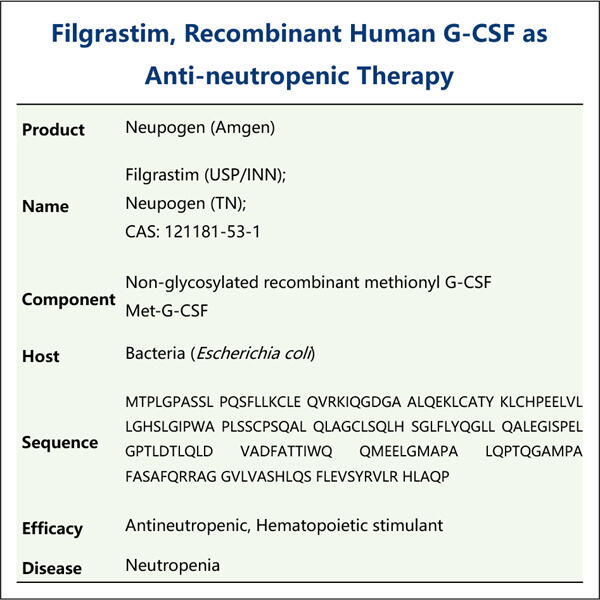
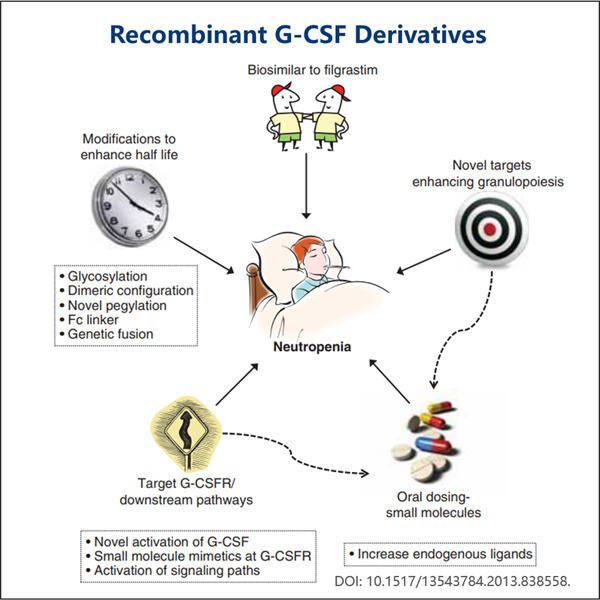
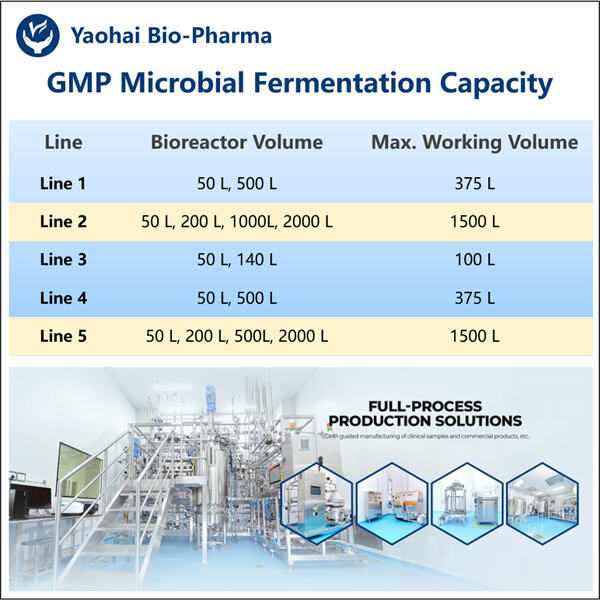
Професионална експертиза и долгогодишно искуство
Јаохаи Био-Фарма, лидер во CDMO за микробиолошки биолошки производи, се наоѓа во Џиангсу. Се фокусираме на микробиолошки произведени терапевти и вакцини кои се GMP G-CSF Производство за управување со здравјето на луѓето, ветеринарството и домашните миленици. Имаме најсовремени платформи за RD, како и производствена технологија која го покрива целиот производствен процес, од развој на микробиолошки соеви, банкарство на клетки, развој на процеси и методи до клиничко и комерцијално производство кое обезбедува успешно производство на нови решенија. Стекнавме големо искуство во био-обработка на микробни клетки. Повеќе од 200 проекти се успешно завршени, а ние ги поддржуваме нашите клиенти во прописите да се пробијат, како што се оние на FDA на САД, како и на ЕУ EMA. Ние, исто така, им помагаме со Австралија TGA и Кина NMPA. Нашето искуство и професионално знаење, како и нашето широко знаење ни овозможуваат брзо да одговориме на барањата на пазарот и да обезбедиме приспособени CDMO услуги.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 НЕ
НЕ
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN