IFN-Gamma ražošanas perspektīvas
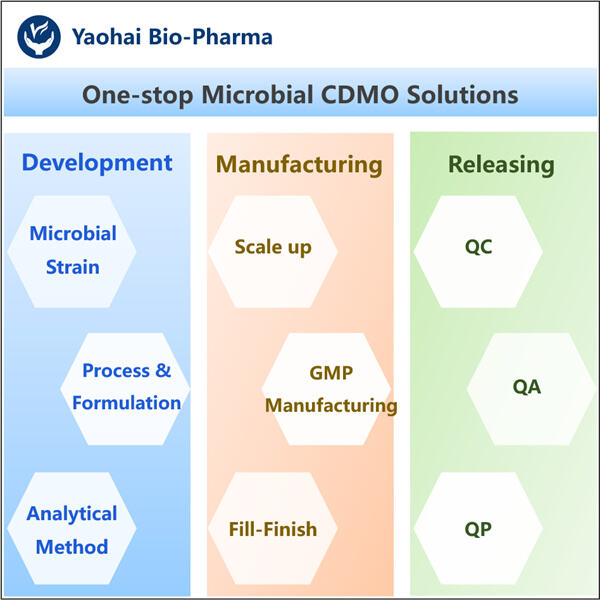
Pēc tam viņi veic dažas attīrīšanas darbības, lai nodrošinātu, ka IFN-Gamma ir tīrs un bez jebkādiem nevēlamiem piemaisījumiem. Tas ir tik svarīgi, jo mēs vēlamies nodrošināt, ka tas, ko sniedzam cilvēkiem, ir drošs un efektīvs. Beidzot tīrais, attīrītais IFN-Gamma proteīns tiek iesaiņots, lai ārsti to izmantotu dažādos ārstniecības pielietojumos.
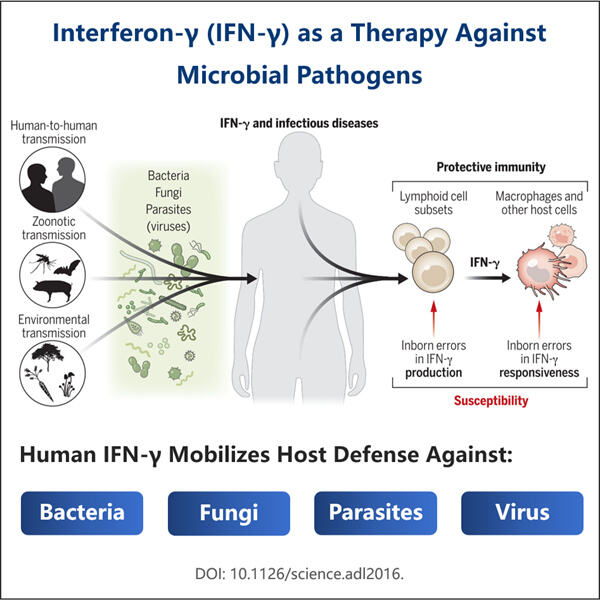
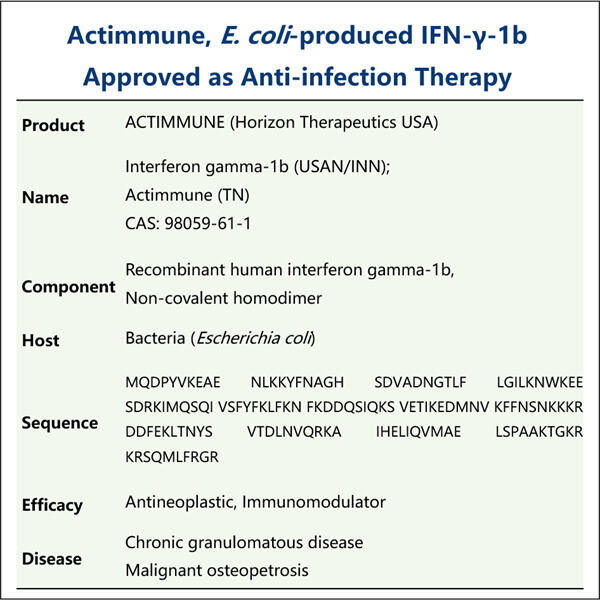
IFN-Gamma ražošana ir būtiska veselības uzturēšanai. Tas ir proteīns, ko lieto tādu slimību ārstēšanai kā vīrusu infekcijas, piemēram, B un C hepatīts, kas var ietekmēt aknas, multiplā skleroze, kas ir neiroloģiska slimība, un daži vēža veidi. Pieaugot cilvēku skaitam, kuriem tiek diagnosticētas šādas slimības, pieprasījums pēc IFN-Gamma pieaug. Yaohai pieliek daudz pūļu, lai mēģinātu izveidot pietiekami daudz IFN-Gamma, lai apmierinātu visu šo pacientu pieprasījumu. Mums ir jābūt pietiekami daudz šī produkta olbaltumvielu, lai ikviens varētu saņemt medicīnisko aprūpi, lai justos labāk."

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NĒ
NĒ
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN