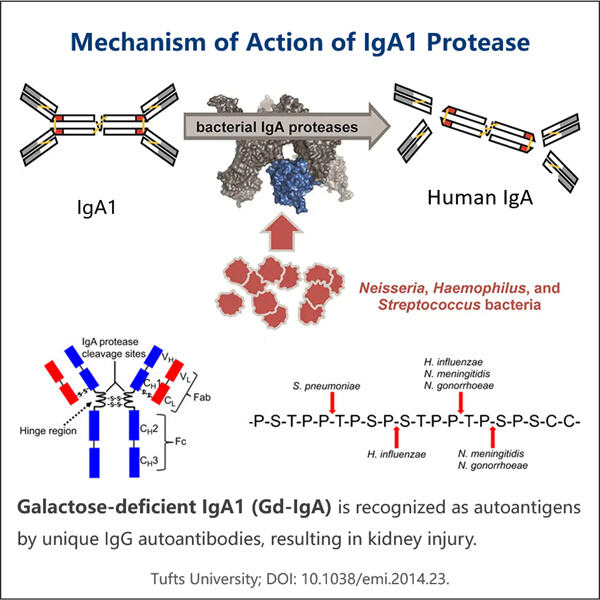
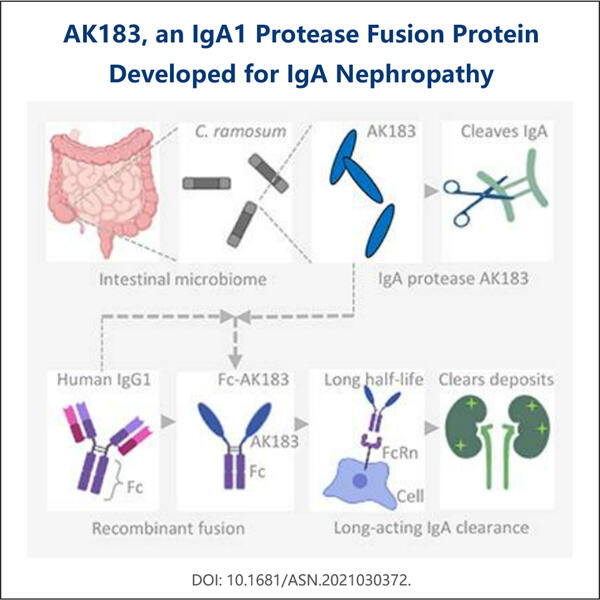
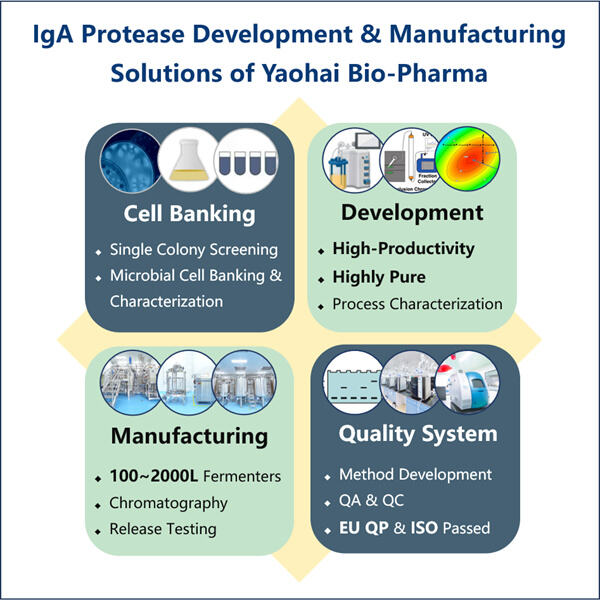
Profesionálna odbornosť a rozsiahle skúsenosti
Yaohai Bio-Pharma, líder v oblasti CDMO pre mikrobiálne biologické látky, sa nachádza v Jiangsu. Zameriavame sa na mikrobiálne vyrábané terapeutiká a vakcíny, ktoré vyrábajú rekombinantnú IgA1 proteázu pre zdravie ľudí, zvierat a domácich zvierat. Máme najmodernejšie platformy RD, ako aj výrobné technológie, ktoré pokrývajú celý výrobný proces, od vývoja mikrobiálnych kmeňov, bunkového bankovníctva, vývoja procesov a metód až po klinickú a komerčnú výrobu, ktorá zabezpečuje úspešnú výrobu nových riešení. Získali sme rozsiahle skúsenosti v oblasti bio spracovania mikrobiálnych buniek. Úspešne bolo dokončených viac ako 200 projektov a podporujeme našich klientov v tom, aby prešli nariadeniami, akými sú napríklad predpisy amerického FDA, ako aj EU EMA. Pomáhame im aj s Austráliou TGA a Čínou NMPA. Naše skúsenosti a odborné znalosti, ako aj naše rozsiahle znalosti nám umožňujú rýchlo reagovať na požiadavky trhu a poskytovať služby CDMO na mieru.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN