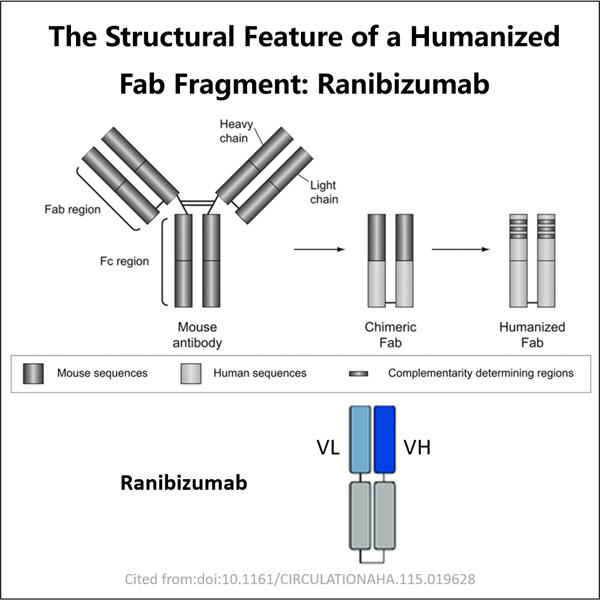
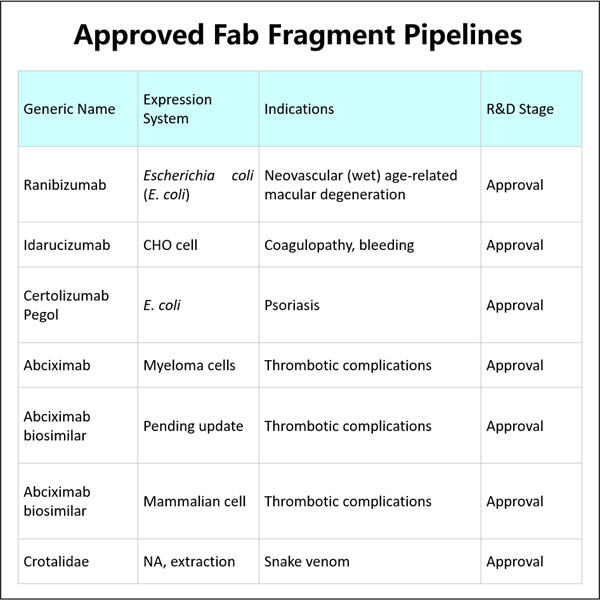
Furtunat Yaohai este un produs bun cu Ranibizumab, medicament special. Este folosit pentru a trata problemele unei condiții legate de ochi și denumită degenerarea maculară legată de vârstă (AMD). Aceasta este un simptom ceea ce este comunmente numit 'vedere legată de vârstă' și, pe măsură ce treci prin ani, va provoca haos în abilitatea ta de a vedea clar. Ranibizumab este produs prin tehnologia DNA recombinant. Cum îl producem în mod sigur și eficient astfel încât cei care ar trebui să-l utilizeze să beneficieze?
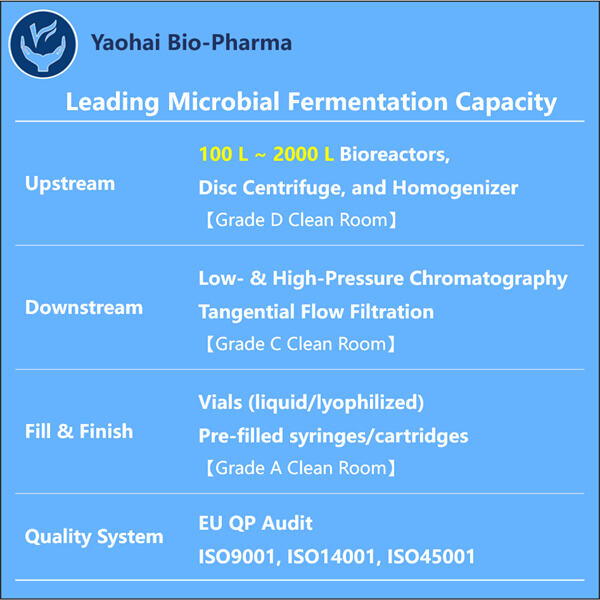
GMP reprezintă Bunele Practici de Fabricație, similare produselor Yaohai, precum Dezvoltare a Procesului de Fragment Fab . Ele sunt de asemenea legi necesare, deoarece ajută la producerea medicamentelor în condiții sigure și conforme pentru oameni. Ranibizumab este fabricat din ceea ce se numesc materii prime. Acestea sunt elementele din care este constituit medicamentul. Fiecare componentă trebuie selectată manual, asigurându-se că este cât mai curată și de cea mai bună calitate. Acest lucru este strict supravegheat, deoarece chiar o mică greșeală ar putea să facă medicamentul mai puțin eficient. Odată aprobat și verificat totul, amestecarea se realizează într-o zonă de clasă 100 sau cameră curată pentru a evita orice contaminante externe.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN