Personalização, eficiência e relação custo-benefício
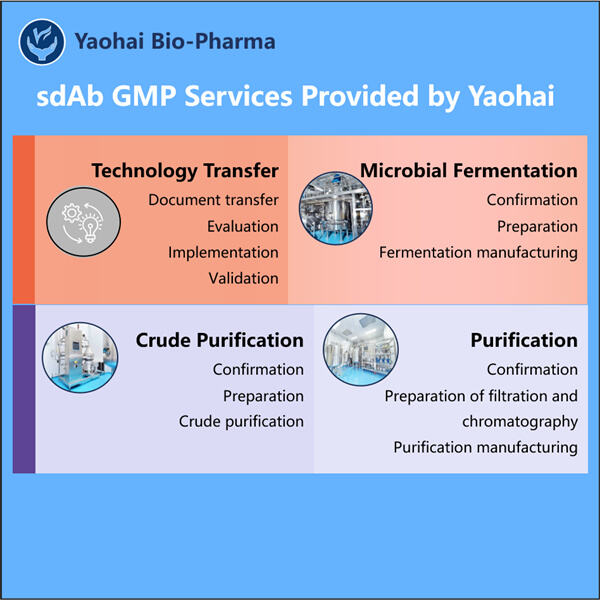
Yaohai Bio-Pharma has experience in the manufacture of biologics derived from microorganisms. We offer tailored RD solutions and manufacturing while minimizing the risk. We have worked with diverse methods, such as GMP sdAb Manufacturing of vaccines (including peptides), growth factors, hormones, and Cytokines. We specialized in multiple microbial hosts, including yeast extracellular and intracellular (yield up to 15 g/L) bacteria periplasmic secretion, soluble intracellular, and inclusion bodies (yield up to 10 grams/L). We also have a BSL-2 fermentation platform to create bacterial vaccines. We specialize in improving processes, increasing product yields, as well as reducing production costs. We have an efficient technology team that guarantees timely and quality project delivery. This allows us to deliver your exclusive products quicker to market.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NÃO
NÃO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN