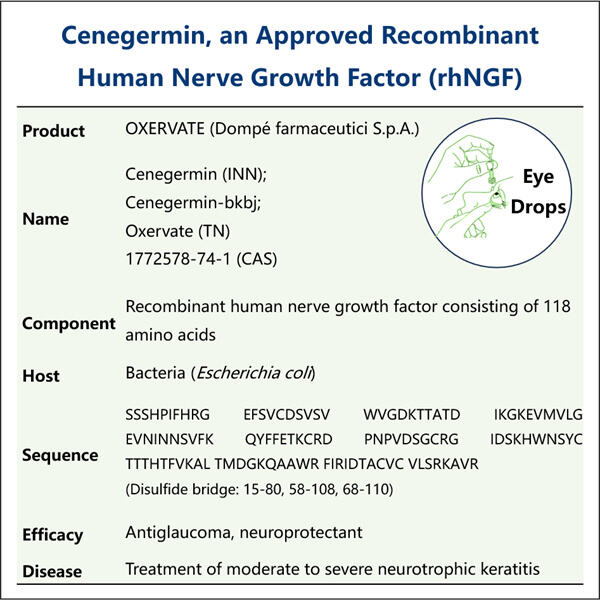
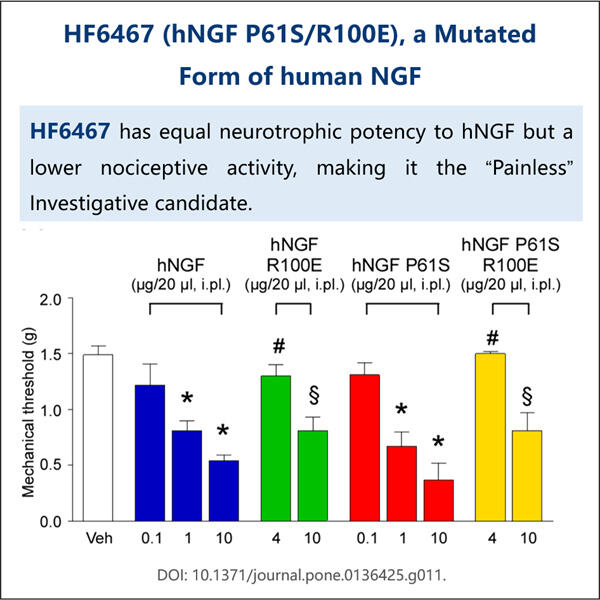
Pritaikymas, efektyvumas ir ekonomiškumas
Yaohai Bio-Pharma turi patirties gaminant biologinius preparatus, gautus iš mikroorganizmų. Siūlome pritaikytus RD sprendimus ir gamybą, sumažindami riziką. Mes dirbome su įvairiais metodais, tokiais kaip rekombinantinis NGF vakcinų (įskaitant peptidus), augimo faktorių, hormonų ir citokinų gamyba. Specializuojamės daugelio mikrobų šeimininkų, įskaitant mielių tarpląstelinių ir tarpląstelinių (išeiga iki 15 g/l), bakterijų periplazminės sekrecijos, tirpių tarpląstelinių ir inkliuzinių kūnų (išeiga iki 10 gramų/l). Taip pat turime BSL-2 fermentacijos platformą bakterinėms vakcinoms kurti. Mes specializuojamės tobulinant procesus, didinant produktų išeigą, taip pat mažinant gamybos kaštus. Turime efektyvią technologijų komandą, kuri garantuoja savalaikį ir kokybišką projektų pristatymą. Tai leidžia mums greičiau pristatyti jūsų išskirtinius produktus į rinką.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NE
NE
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN