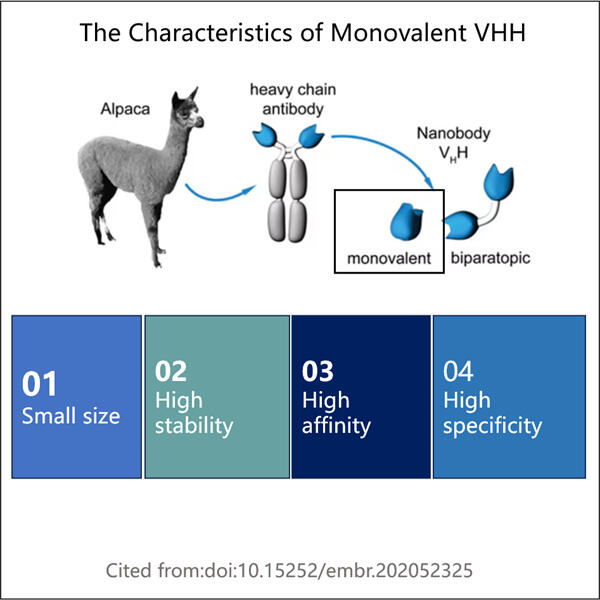
Pentru a fi clar, Yaohai de ani de zile produce o substanță cunoscută sub numele de VHH monovalent. Ați realizat până la această halbă? Acolo folosesc mașini exotice și tehnologie de ultimă oră pentru a produce produse de clasă mondială. Cât despre tine, s-ar putea să te întrebi ce înseamnă VHH monovalent? Monovalent: Termenul „monovalent” se referă la unul, iar VHH înseamnă Variable Heavy Chain Domain. Un fragment de anticorp, o proteină care ajută la stimularea sistemului nostru imunitar. VHH monovalent este diferit, deoarece poate detecta doar anumite elemente din corpul nostru, precum și antigene. Antigenele sunt un fel de voce pentru a face sistemul nostru imunitar să învingă infecțiile. Acest lucru face GMP VHH Fragment Manufacturing un instrument puternic pentru a fi folosit în cercetare și tratamente medicale care ne pot aduce beneficii pe noi, ființele umane. Acesta trece prin câțiva pași pentru a-l realiza, care sunt necesari pentru a se asigura că funcționează eficient.
În prepararea acestuia sunt implicați mai mulți pași, iar fiecare pas este esențial pentru a produce VHH monovalent. Primul pas este ca oamenii de știință să aleagă un animal gazdă, cum ar fi o vacă, o lamă sau o cămilă. Ei administrează un antigen unic animalului, astfel încât acesta să poată produce anticorpi. Mai simplu spus, anticorpii sunt ca gardienii corpului! Animalul este imunizat cu un antigen, substanța împotriva căreia organismul trebuie să se protejeze prin producerea de anticorpi. Ulterior, anticorpii sunt colectați pentru etapele ulterioare.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NU
NU
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN