Tilpasning, effektivitet og kostnadseffektivitet
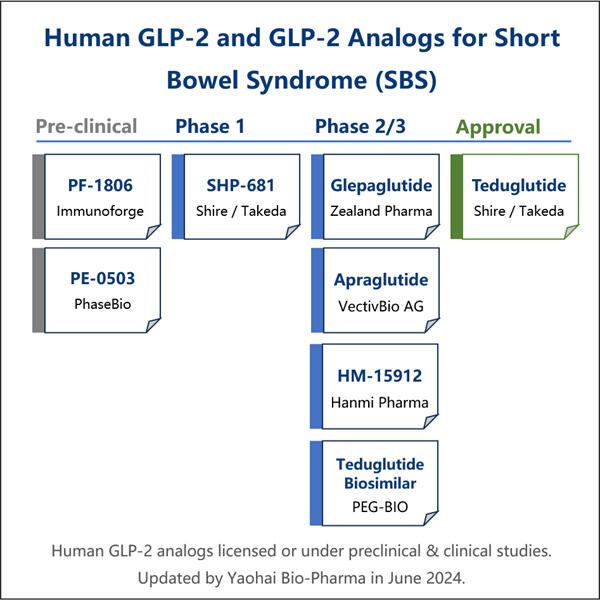
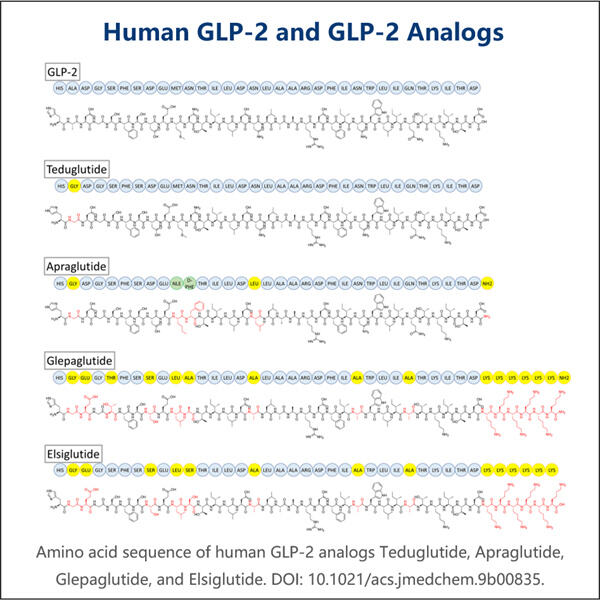
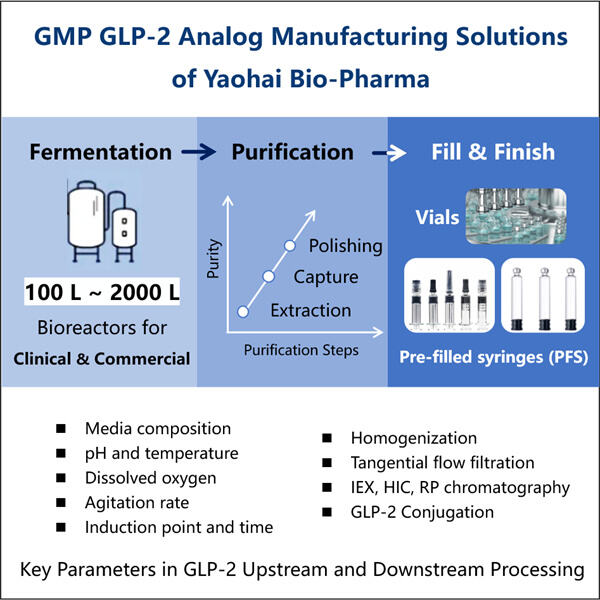
Yaohai Bio-Pharma is experienced in the development of microbial-derived biologics. We offer customized RD as well as manufacturing solutions, while making sure that there are no risks. We have worked on diverse modalities such as subunit-based recombinant vaccines, GLP-2 Analogue Biomanufacturing, cytokines, growth factors, single domain antibodies, enzymes, plasmid DNA, the mRNA, and other. We are experts in a variety of microorganisms, including yeast extracellular and intracellular secretion (yields up to 15g/L) as well as bacteria intracellular soluble and inclusion body (yields as high as 10g/L). We have also developed the BSL-2 fermentation platform to create bacteria-based vaccines. We have a track record of improving production processes, thereby increasing yields and decreasing costs. With a highly efficient technology team, we ensure timely and quality project delivery and bring your products to market faster.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NEI
NEI
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN