Precise Manufacturing Techniques for Optimal Efficacy
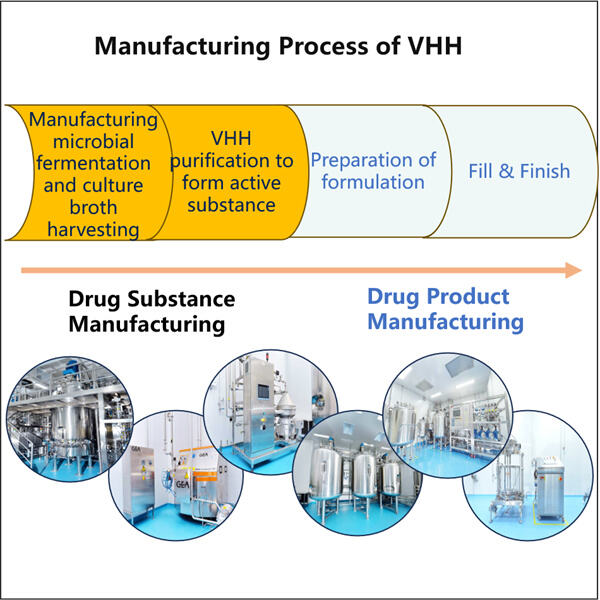
Don't worry, Yaohai watches the making process every step of the way to ensure everything is done perfectly and safely just so you guys do not have to think about anything. Most of the operations that this entails encompass the processing of materials, the blending of components, bottle stuffing, medicine packaging or relabeling. All of these GMP Plasmid DNA Tillverkning steps are important because they all play a part in the final product we get. It pays careful attention to each step to ensure that the medicine be properly made from beginning till end.
Among these methods, one has to be cautious with the quantity of ingredients used, keeping a check on moisture levels, temperature control and cleaning of the equipment used. The process has everything to do with ensuring the correct amount of medicine is manufactured. If not done correctly, one of any one of these steps could potentially ruin the efficacy of the medicine.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NEJ
NEJ
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN