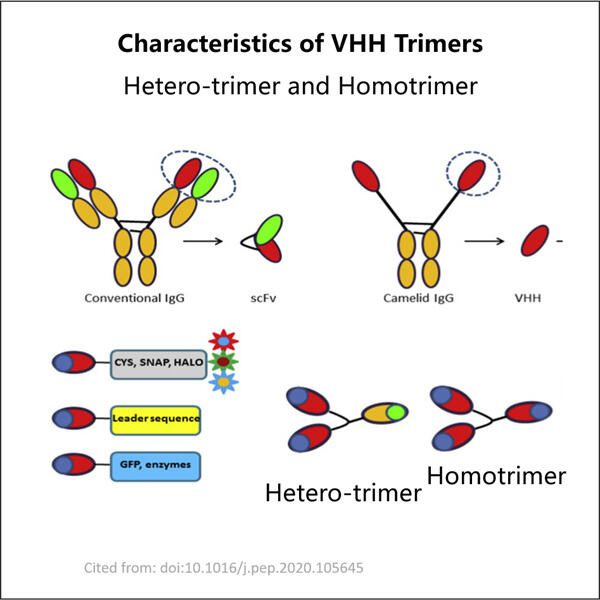
Hos Yaohai er vi spesielt flinke til å produsere noe som kalles VHH-trimer-antistoffer. Disse spesielle antistoffene er viktige fordi de behandler mange forskjellige sykdommer som finnes hos mennesker. VHH-trimerantistoffer består av tre små domener. Disse seksjonene er kjent som VHH-domener, og de binder seg til kroppens spesifikke mål i denne konfigurasjonen. Dette er hvordan de bidrar til å bekjempe sykdom og holde mennesker friske.
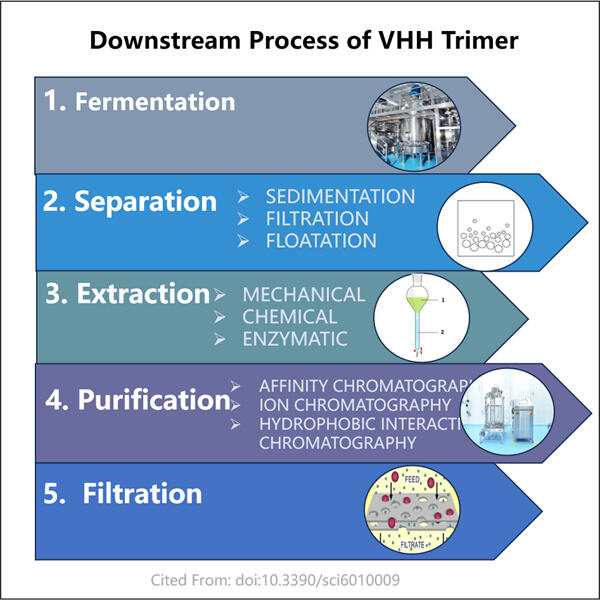
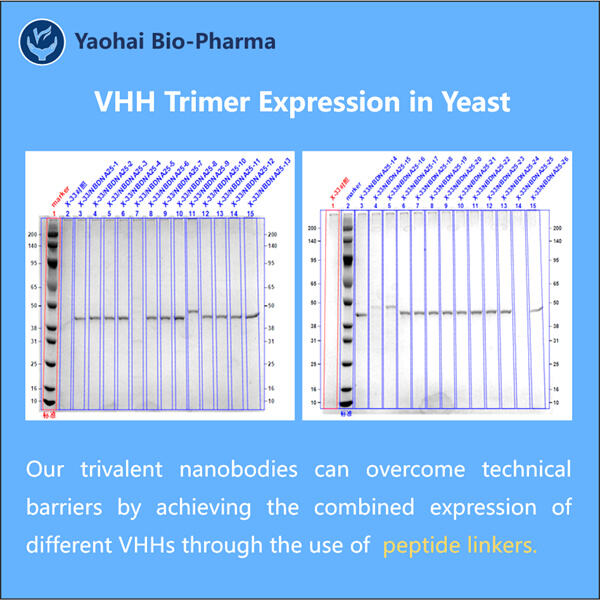
Tidligere var det en møysommelig prosess å generere VHH-trimer-antistoffer. Det var vanskelig og veldig kostbart. Så folk som hadde forseggjorte maskiner og visste hvordan de skulle arbeide dem gjorde alt arbeidet. Hos Yaohai har vi imidlertid oppdaget raskere, enklere og rimeligere måter å lage disse antistoffene på. Dette representerer et stort hopp fremover, ikke bare for oss, men for pasientene som trenger disse behandlingene.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NEI
NEI
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN