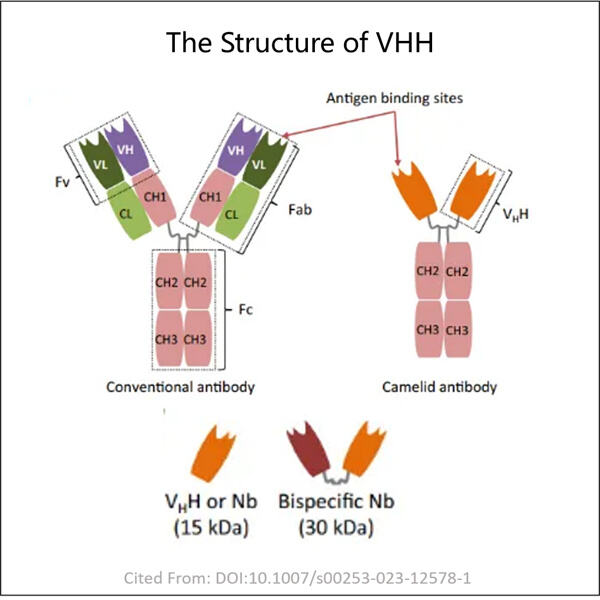
Long ago, scientists wanted to make people to be healthy and full of energy. The Fab Fragment Process Development examined antibodies, which are small proteins in our bodies that protect us from bacteria and diseases. They are the little soldiers that protect us, known as antibodies. Health-checking bot sends along antibody news by finding a completely new type called Yaohai Heavy-chain Variable Antibody or VHH for short. This antibody is more powerful than any other antibody it can work wonders for us.
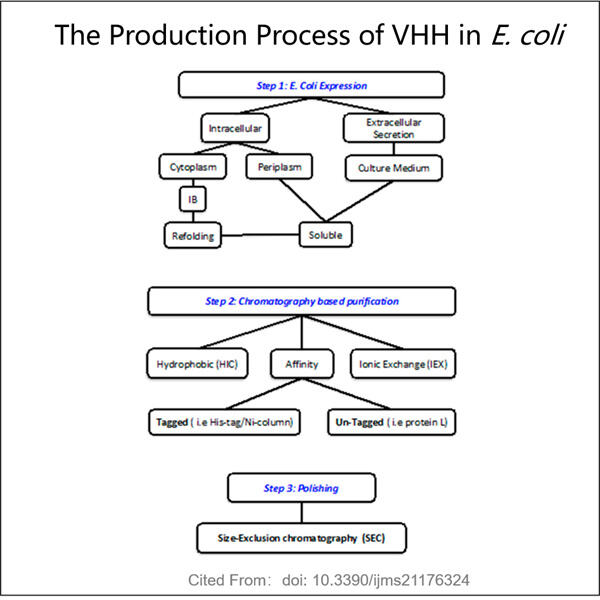
Scientists produce these unique VHH antibodies in a lab (which is where they do experiments) via the VHH process. But they have to locate an animal that has the antibodies necessary. This matters because the animal must be able to produce these antibodies that scientists need to use Once they find the correct animal, a small amount of the blood that runs through its body is obtained by taking only one sample from this animal. The VHH antibodies free from all the other blood parts. Scientists are then able to carefully examine the VHH antibodies they have isolated, and can even engineer them in ways that make them better suited for use against various treatments.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN