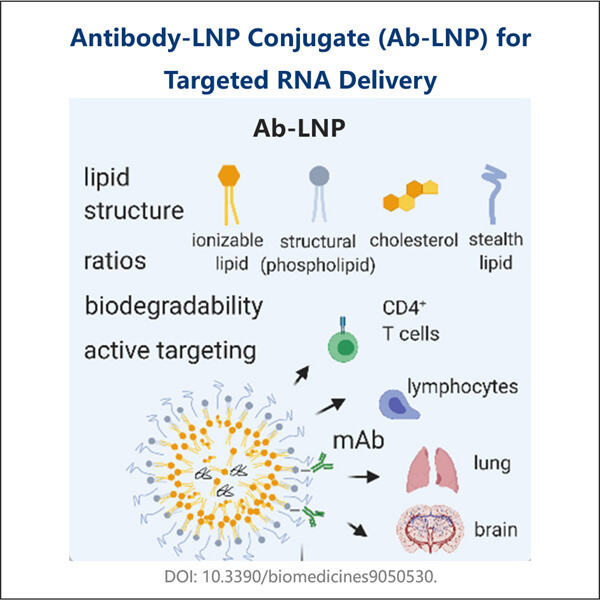
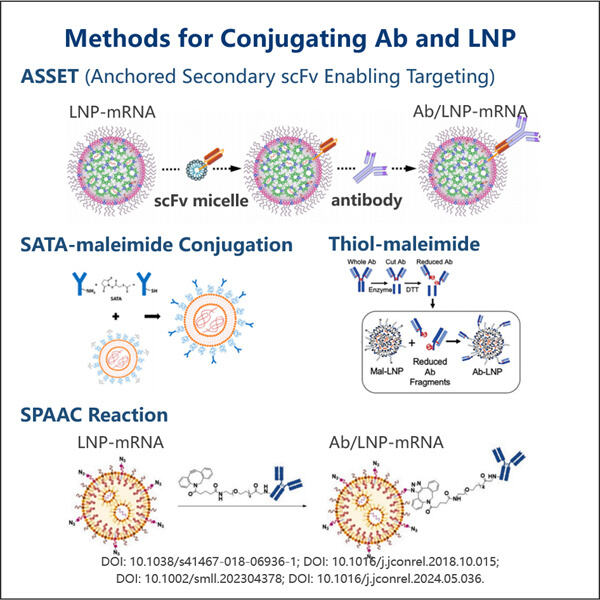
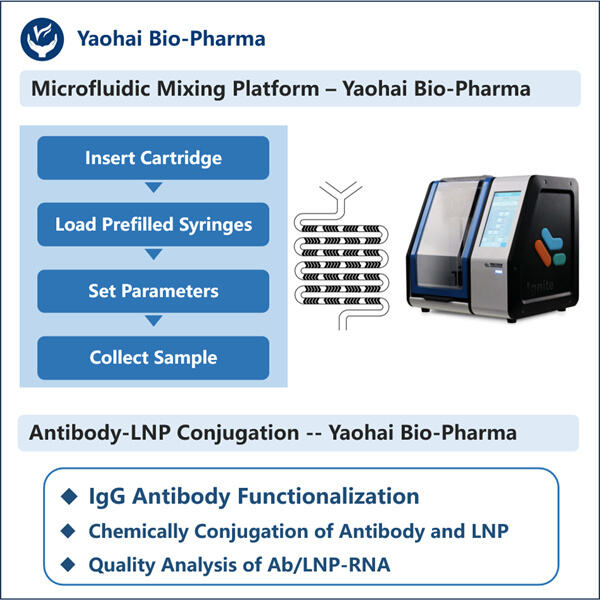
Customization, Efficiency & Cost-Effectiveness
Antibody-LNP Conjugate Preparation has experience in manufacturing biologics that are that are derived from microorganisms. We provide tailored RD as well as manufacturing solutions, while minimising the risk. We have experimented with a variety of techniques, such as recombinant cellular subunits of vaccines (including peptides), growth factors, hormones and the cytokines. We have specialized in multiple microorganisms like yeast extracellular and intracellular secretion (yields up to 15g/L) and bacteria intracellular soluble and inclusion bodies (yields up to 10g/L). We also have the BSL-2 fermentation platform to develop bacterial vaccines. We are experts in improving processes, increasing product yields, and decreasing production costs. With an effective technology team, we ensure timely and quality project delivery and bring your products to market faster.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN