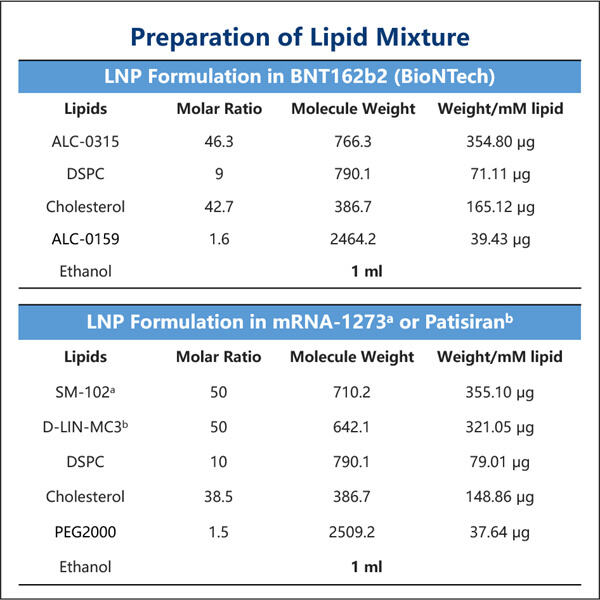
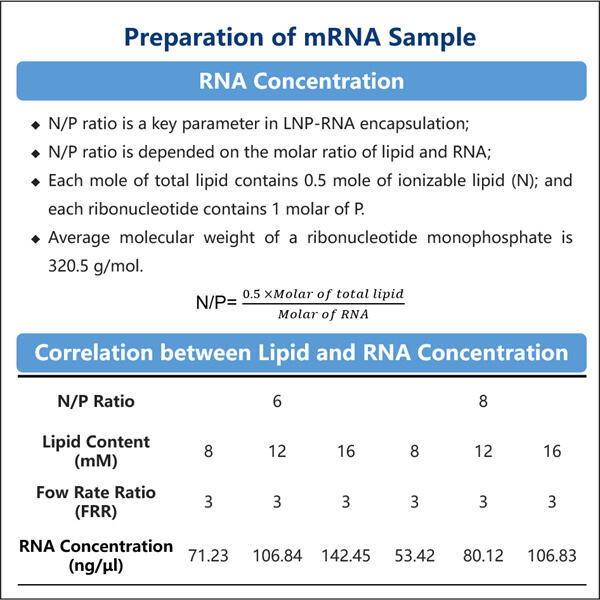
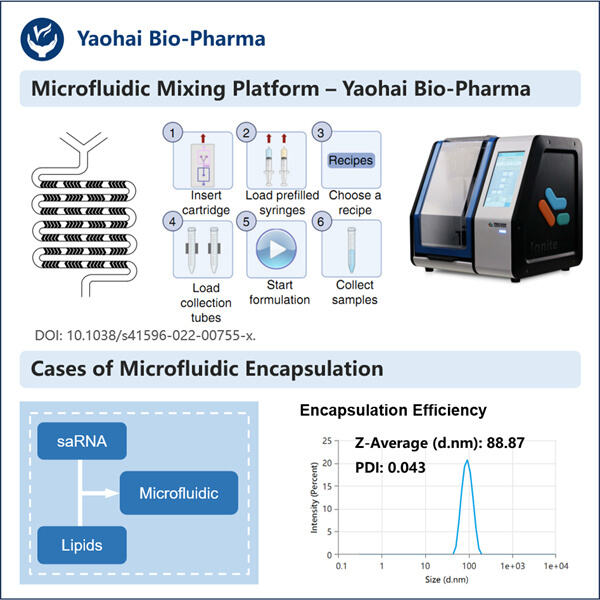
LNP encapsulation technology is a method that uses a type of fat molecule, known as a lipid nanoparticle (LNP), to deliver the medicine into your cells. Lipid nanoparticles are super small, and they can be made of different fats as per the medicine they are supposed to carry. Lipid nanoparticles can be thought of as small medicine-loaded trucks, carrying the drugs within.
LNP capsules are a small bubble that encases the medicament and shields it while it moves through your body. This is extremely critical as it makes sure your medicine reaches the right place without damaging it or simply getting destroyed by your body defense system. The LNP capsule holds the medicine in much the same way as a bubble encases a toy.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN