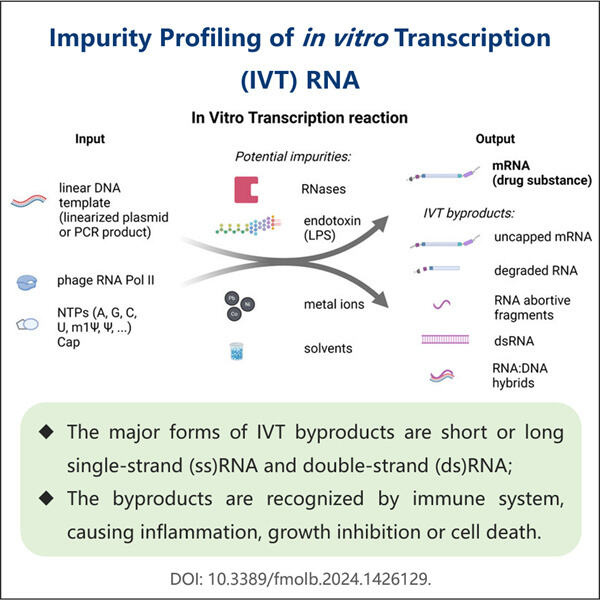
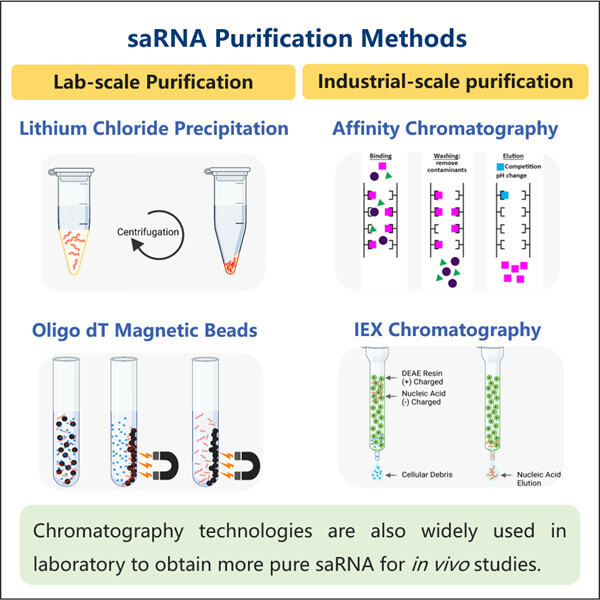
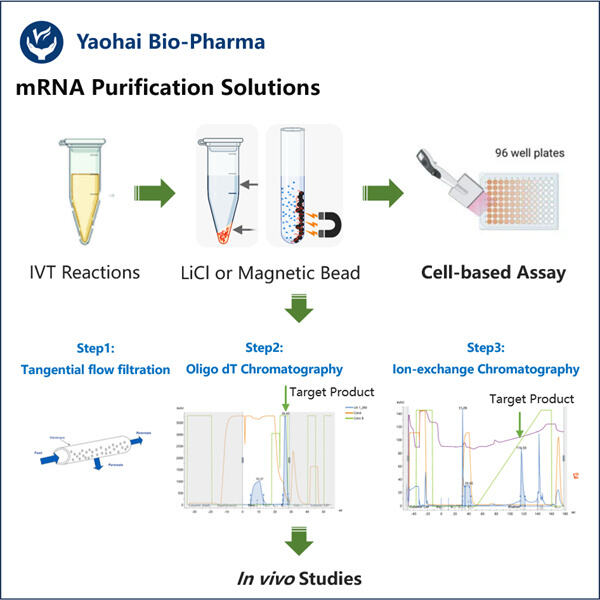
Membaiki Kebanyakan saRNA dengan Protokol Pemurnian yang Boleh Dipercayai
Kami telah dengan teliti merancang program pembersihan dan sanitasi untuk para penyelidik supaya anda tidak perlu risau semasa bekerja mendapatkan kualiti terbaik saRNA. Kami menggunakan prosedur terkini dan teknologi canggih untuk memastikan bahawa saRNA sepenuhnya disisihkan daripada sebarang molekul gangguan atau kekotoran. Kejadian pencemaran silang sangat minimum, dan ini sangat penting untuk mengekalkan integriti dan keupayaan saRNA bagi penyelidikan anda.
Proses pembersihan yang keras ini sangat kritikal kerana saRNA mungkin berinteraksi dengan bahan kimia lain. Maka, kecekapan dan kebolehpercayaan hasil dalam eksperimen akan menurun. Walau bagaimanapun, sistem kami menjamin bahawa saRNA yang diberikan kepada para penyelidik adalah bersih dan murni sebanyak mungkin dan, oleh itu, memaksimumkan potensinya menuju kepada kajian terobosan. Samada dalam biologi molekul, pembangunan vaksin, atau penemuan terapi. Protokol pembersihan kami menjamin sampel anda untuk kualiti penyelidikan.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN