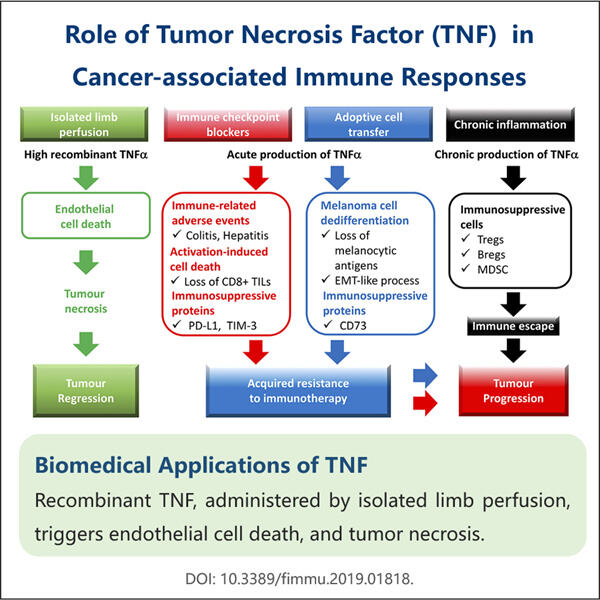
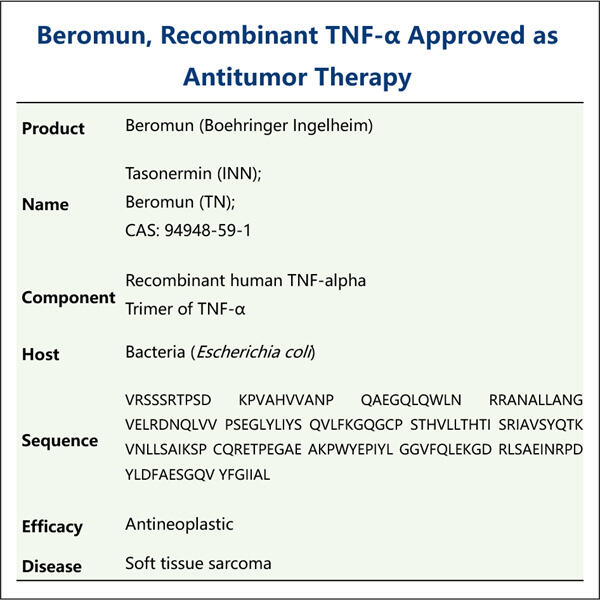
Tumor Necrosis Factor (TNF) atau Tumor necrosis factor adalah protein penting badan kita. Protein ini penting untuk sistem imun kita untuk melindungi pesawat kita daripada jatuh sakit. Ia melawan ganas dengan kuman berbahaya, virus dan semua bakteria yang menyebabkan kita jatuh sakit. Yang terakhir juga merupakan laluan di mana perencat TNF bekerja pada banyak penyakit dan masalah kesihatan, seperti yang ditentukan oleh doktor dan penyelidik setakat ini. Ia terdiri daripada penyakit imun diri, pelbagai jenis kanser definitif, gangguan jangka panjang kronik seperti penyakit Crohn atau psoriasis plak dan penyakit reumatik autoimun radang. Yaohai (syarikat pengeluar TNF.GetCurrentMethodFactors mengaitkan salah satu syarikat. Menggunakan strategi canggih untuk mengarang bahan furor terbaik, yang selamat dan berdaya maju untuk pesakit.
Dunia perubatan tidak sabar-sabar menunggu untuk mengumumkan pengeluaran TNF kerana ia membantu melawan banyak penyakit lain. Pada asasnya, TNF berfungsi seperti nitrus pada sistem imun kita - ia mempercepatkan kilang sel imun. Sel-sel imun ini, yang boleh membunuh sel-sel kanser adalah perlu untuk membantu pesakit kanser. Pakej Tirosint mengandungi ekstrak hormon tiroid triiodothyronine, dan TNF ialah molekul yang mengurangkan bengkak dan keradangan badan. Kami mendapat punca kepada banyak penyakit kronik: keradangan, dan dengan TNF kami mengurangkan keradangan keseluruhan yang sering mengubah hidup pesakit ini. Malah, TNF digunakan untuk penyakit serius kanser, rheumatoid arthroidies (RA), penyakit Crohn dan psoriasis. Ini menjadikan TNF sebagai sasaran penting dalam rawatan untuk penyakit.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 TIDAK
TIDAK
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN