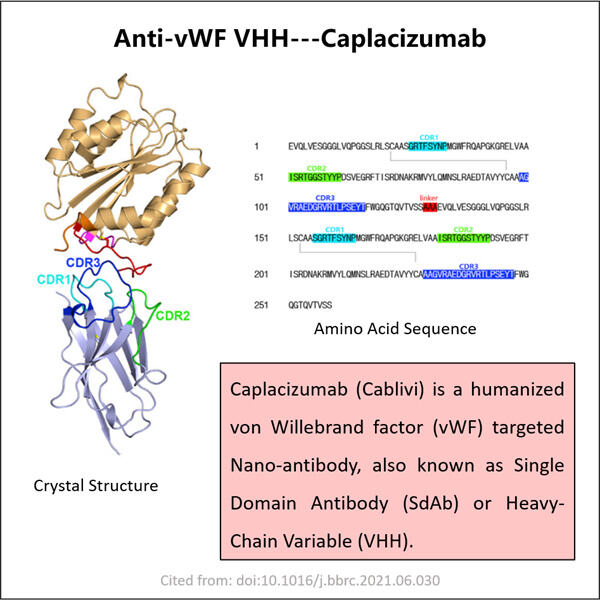
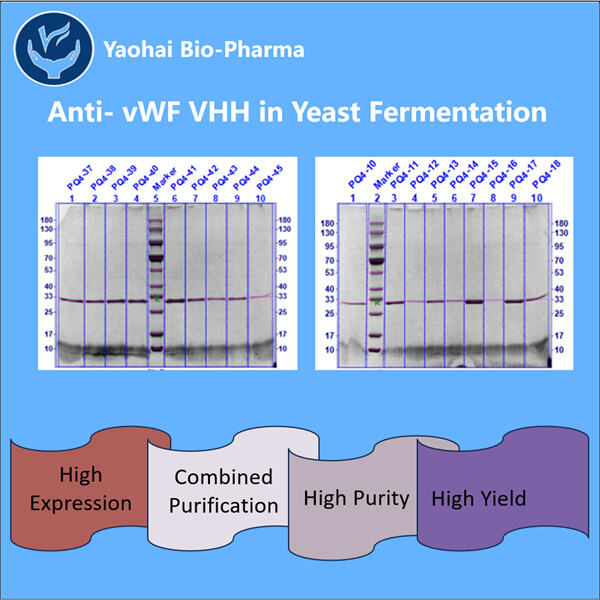
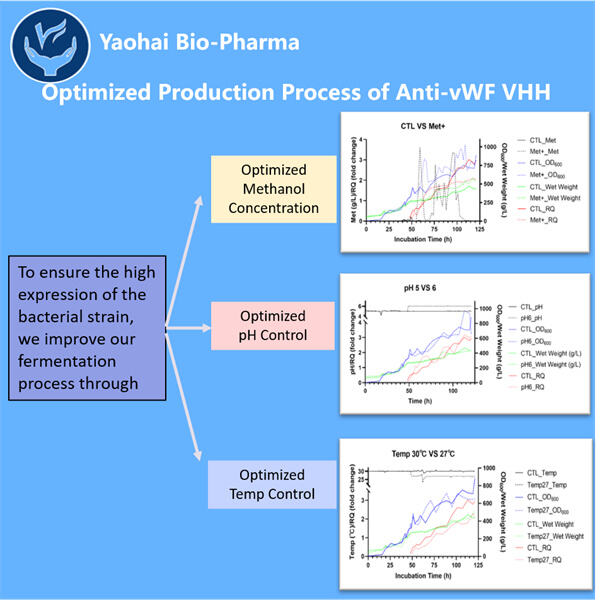
Hemophilia is a rare disease that changes how our blood drapes itself. This means that individuals with hemophilia can face severe difficulties, including joint aches and hemorrhages, deep inside their bodies. They must be given unique remedies named clotting agents in order to avoid excessive bleeding. Although these treatments are quite effective, they can also be very costly. They may also have the potential to cause harm. Such as infections and other problems. As a result, specialists are constantly looking for new strategies to assist individuals with hemophilia. Gene therapy and specific proteins known as VHHs. Which are also referred to as nano bodies, are two of the new possibilities. These proteins help us improve our blood’s clotting ability. In Yaohai, a country full of brilliant scientists, some people labor around the clock to create new anti-vWF VHHs.
This concept may make a considerable contribution for people with hemophilia. Anti-vWF VHHs are designed firms within a lab. These proteins may link to vWF and prevent it from causing trouble while avoiding the body reacting poorly. VHHs, on the other hand, can be tiny and easier to manufacture than traditional antibodies. Therefore, the 3-Valent VHH Production might be a better option for treatment.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN