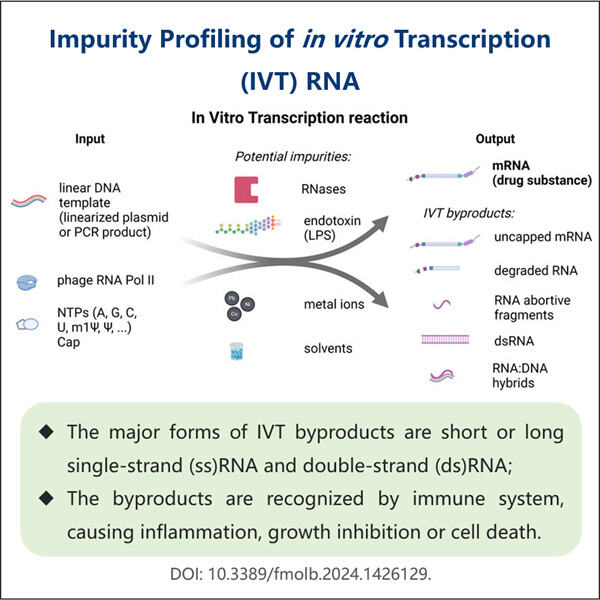
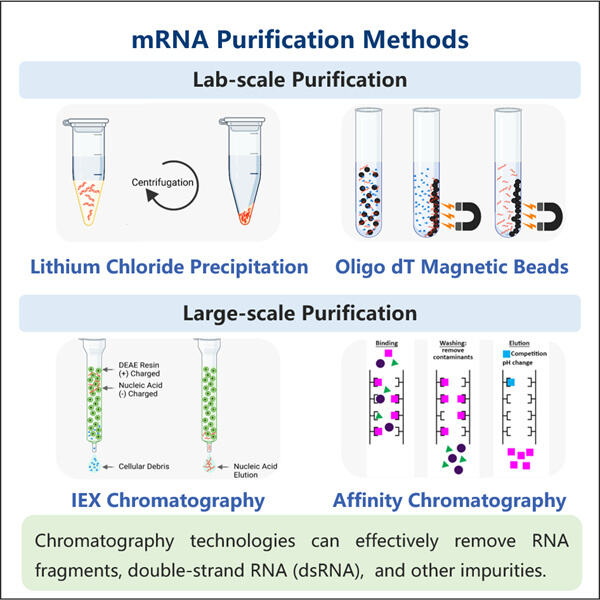
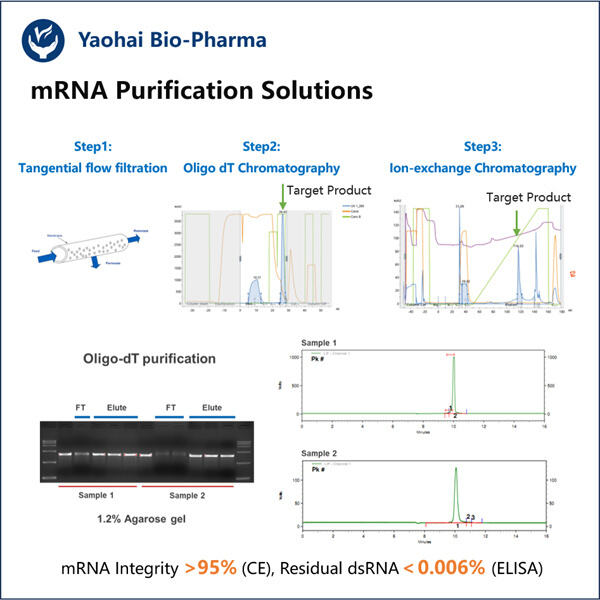
A Step-by-Step Purification Guide
First, prepare your first sample. This required the addition of a lysis buffer, any special liquid. The liquid is necessary as it ruptures the walls of the cells to allow the mRNA to be released.
You will now seed your sample with small magnetic beads. These beads are of huge significance because they will stick to the present mRNA in your sample. So this means the beads to go along with the mRNA and they are going to work together.
The beads and mRNA are now ready to be washed, as well as the in vitro Transcript Protocol made by Yaohai. The method to this is all by rinsing with a wash liquid. This wash liquid is very important as it helps remove anything that does not belong there, thus providing a cleaner mRNA sample to work with.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN