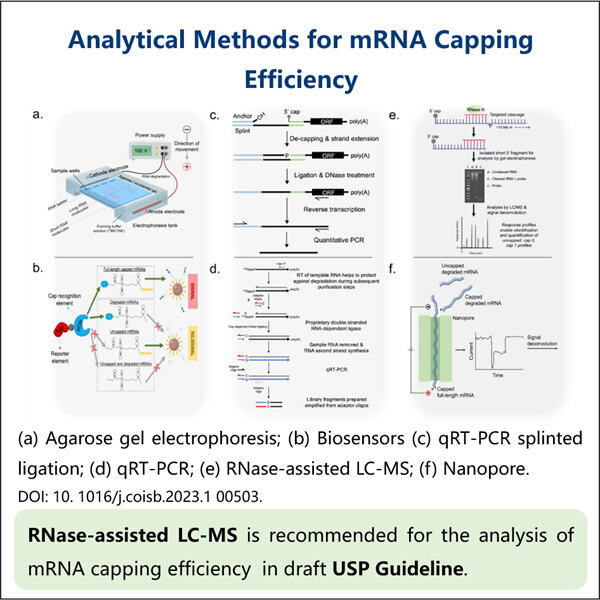
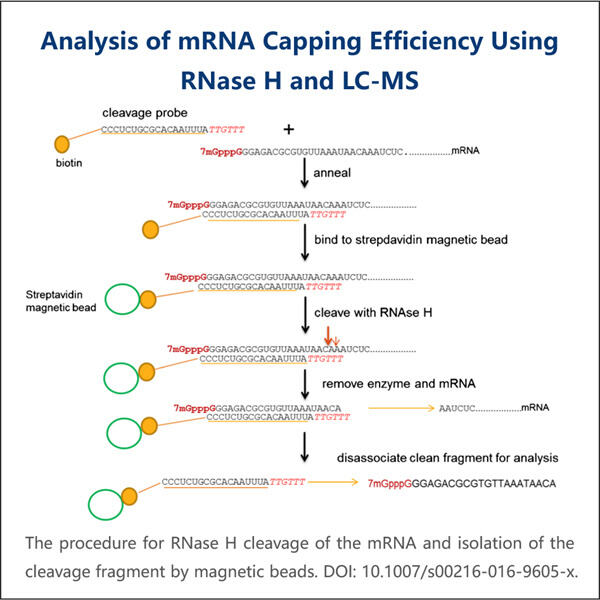
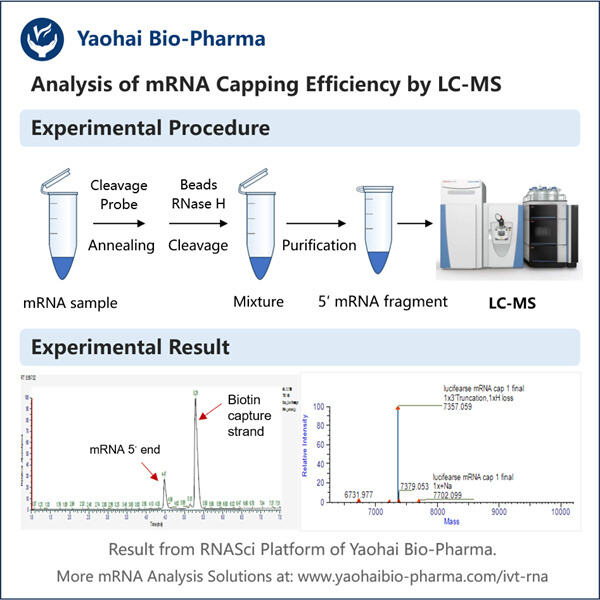
Pernahkah kamu mendengar tentang sesuatu yang disebut mRNA? Asam ribonukleat perantara — ia membantu memproduksi protein. Protein adalah mesin kecil yang melakukan banyak pekerjaan dalam sel kita. Ilmuwan mempelajari mRNA untuk memahami seberapa baik ia memproduksi protein-protein ini. Efisiensi hanyalah kata lain untuk seberapa baik suatu proses bekerja. Penutup adalah bagian penting dari mRNA. Penutup adalah 'topi' kecil di ujung 5′ dari mRNA. Ia menstabilkan mRNA, dengan demikian menjaganya tetap terikat dan mencegahnya terurai terlalu cepat. Penutup juga memberi tahu mRNA ke mana harus pergi dalam sel agar dapat bekerja. Untuk menguji kualitas penutup, ilmuwan melakukan analisis efisiensi penutupan mRNA.
Pada titik ini, Anda mungkin bertanya-tanya mengapa penting bagaimana baiknya tutup (cap) dibuat. Dan itu bisa merusak banyak hal jika tutup tidak dibuat dengan benar. mRNA mungkin tidak stabil jika tidak memiliki tutup yang baik. Masalahnya adalah, ini berarti bahwa mRNA tidak akan bertahan cukup lama untuk memproduksi banyak protein, dan kemudian akan ada masalah dalam sel. Dalam beberapa kasus, mRNA bahkan mungkin salah tempat dalam sel dan tidak menghasilkan protein yang tepat, seperti juga pada Yaohai's. Reporter Gene mRNA . Jadi penting bagi para ilmuwan untuk memahami bagaimana cap bekerja. Dengan melakukan ini, mereka dapat membantu memperbaiki masalah-masalah tersebut dan meningkatkan cara kerja sel kita. Peneliti juga dapat menggunakan pengukuran efisiensi pencapan mRNA untuk mendesain obat-obatan baru. Sebagai contoh, jika mereka menemukan bahwa cap tidak bekerja dengan baik dalam kondisi tertentu, mereka dapat bekerja untuk memperbaikinya dan meningkatkan cap.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN