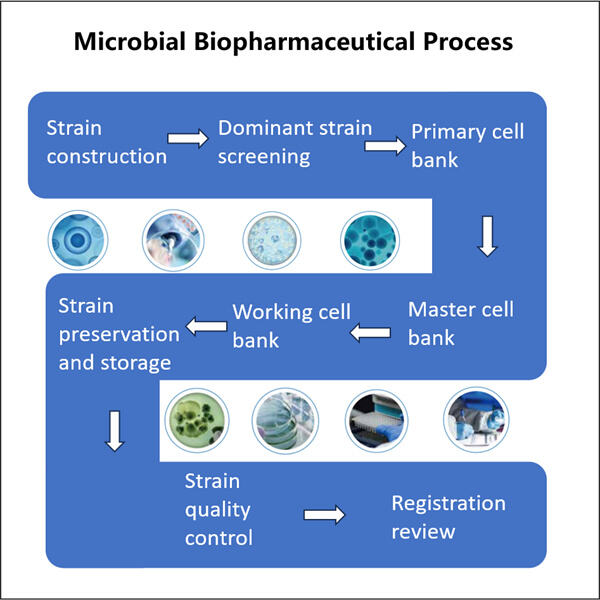
Obat-obatan ini bermanfaat dan memang, esensial untuk menjaga kesehatan manusia: hal ini dimungkinkan oleh Proses Fermentasi Mikroba . Proses-proses ini membuat obat dengan bakteri, ragi, dan jamur. Mereka biasanya datang dalam botol berwarna oranye dan merupakan obat yang diresepkan dokter ketika seseorang sakit untuk membantu mereka sembuh. Contoh yang baik dari ini adalah banyak obat yang digunakan orang sebenarnya diperoleh dari organisme-organisme tersebut.
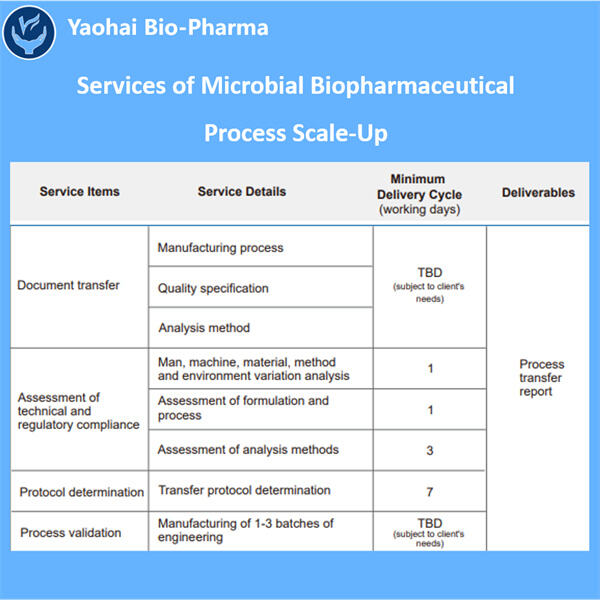
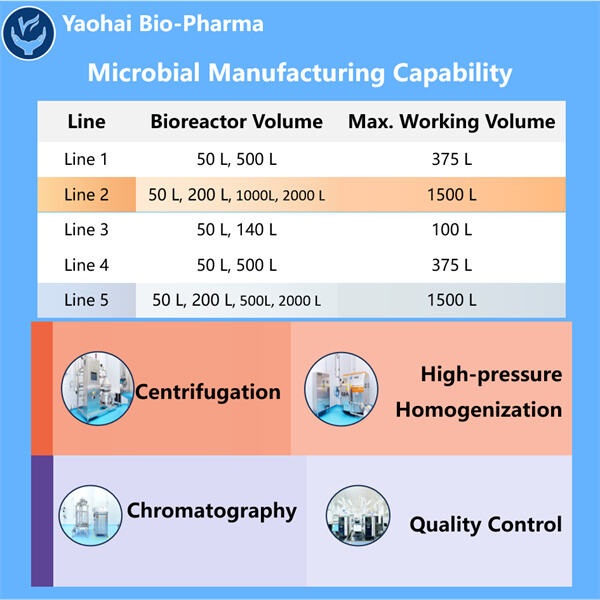
Pembuatan Mikroba untuk Pasokan Klinis mengambil sedikit obat dan membuat jauh lebih banyak, yaitu BANYAK LEBIH BANYAK. Namun, ketika kita membuat lebih banyak obat, lebih banyak orang dapat menggunakannya. Ini mengingatkan saya betapa beragamnya — dan dalam beberapa hal, tak terbatas — pilihan kita untuk berkomunikasi satu sama lain sekarang, yang agak mirip dengan memasak sepotong besar sup agar kita semua bisa mendapatkan lebih dari sekadar sedikit. Jika kita hanya membuat sedikit obat, secara struktural, sangat sedikit orang yang bisa mendapatkannya, dan itu tidak melayani semua orang yang membutuhkannya. Oleh karena itu, penskalaan adalah cara utama untuk memastikan bahwa semua orang yang membutuhkan perawatan dapat memperolehnya.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN