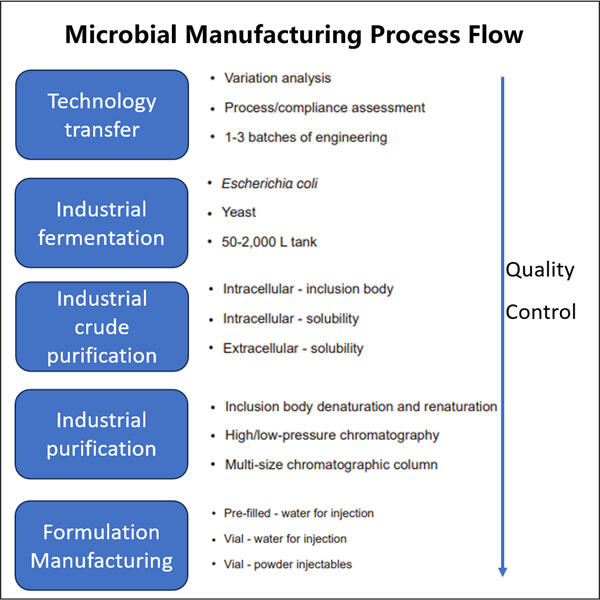
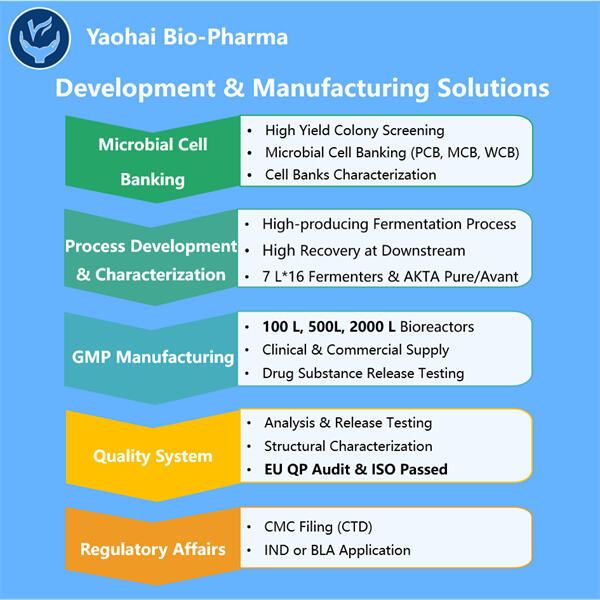
Kemampuan Profesional & Pengalaman Luas
Yaohai Bio-Pharma adalah CDMO mikrobiologi terkemuka. Fokus kami telah berada pada produksi mikroba untuk Manufaktur Mikrobiologis untuk Penyediaan Komersial dan vaksin untuk manusia, veteriner, dan pengelolaan kesehatan hewan peliharaan. Kami memiliki platform R&D dan metode manufaktur yang canggih yang mencakup seluruh prosedur mulai dari pembuatan strain mikroba dan perbankan sel, hingga pengembangan proses dan metode untuk produksi komersial dan klinis serta implementasi solusi terdepan. Selama bertahun-tahun, kami telah memperoleh keahlian yang luas dalam pemrosesan biologi menggunakan sumber mikroba. Kami telah berhasil menyelesaikan lebih dari 200 proyek di seluruh dunia dan membantu klien kami dalam menavigasi regulasi dari US FDA, EU EMA, Australia TGA, dan China NMPA. Karena keahlian dan pengetahuan kami, kami dapat merespons dengan cepat terhadap kebutuhan pasar dan menawarkan layanan CDMO yang disesuaikan.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN