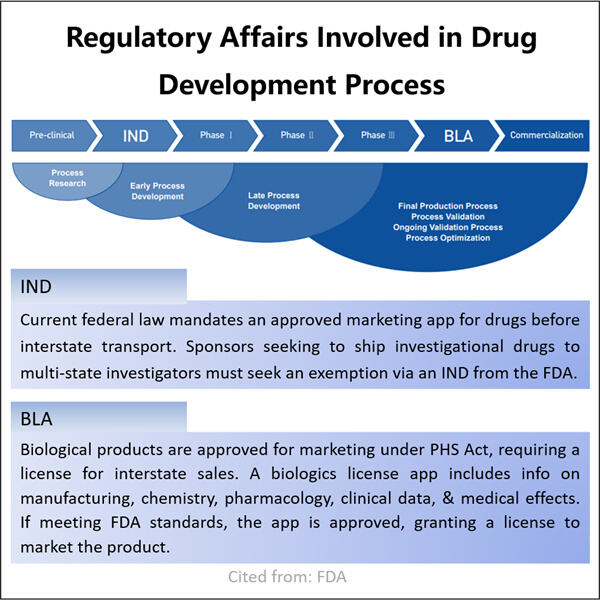
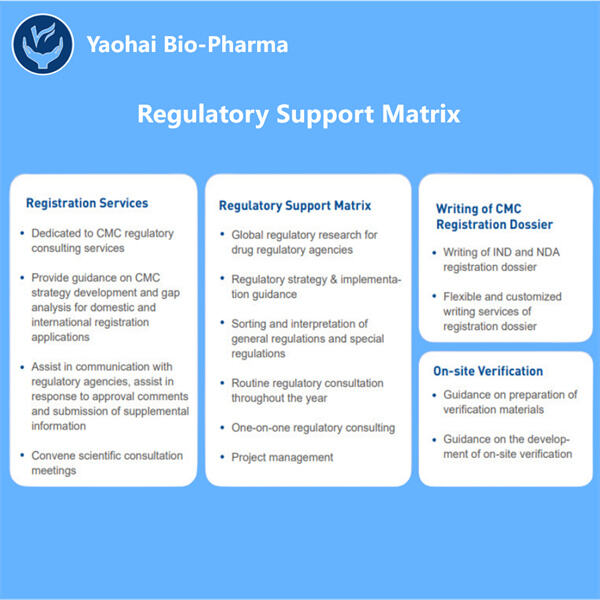
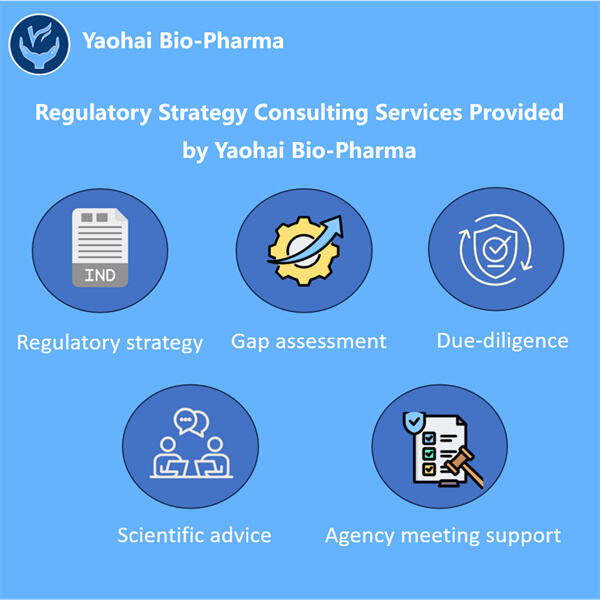
Menjamin Kepatuhan di Pasar Biologis
Setelah memperoleh persetujuan untuk menjualnya, bukan hanya soal menjual obat biologis. Obat harus dibuat dan dijual di bawah regulasi yang ketat. Yaohai Pengembangan Proses Pemurnian Biologis adalah aturan yang ada untuk memastikan bahwa setiap produk obat biologis diproduksi dengan aman dan berkali-kali memenuhi standar kualitas tinggi. Yaohai adalah bisnis yang sangat berorientasi pada kepatuhan, bertujuan untuk mematuhi aturan-aturan ini secara tepat. Dengan cara ini, mereka dapat memastikan bahwa setiap produk obat biologis mereka dibuat dan dipasarkan secara efektif.
Obat biologis, bagaimanapun, menawarkan potensi besar bagi pasien dengan kebutuhan perawatan kompleks tertentu. Ini bisa berarti merancang obat-obatan untuk menargetkan molekul yang terkait dengan penyakit atau kondisi tertentu — dengan kata lain, mereka memiliki potensi untuk menjadi banyak terobosan dalam mengobati berbagai masalah kesehatan. Yaohai menyadari nilai dari obat biologis dan berkomitmen untuk membawa obat inovatif baru kepada kebutuhan medis yang belum terpenuhi.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN