Επαγγελματική Ειδικότητα & Εκτεταμένη Εμπειρία
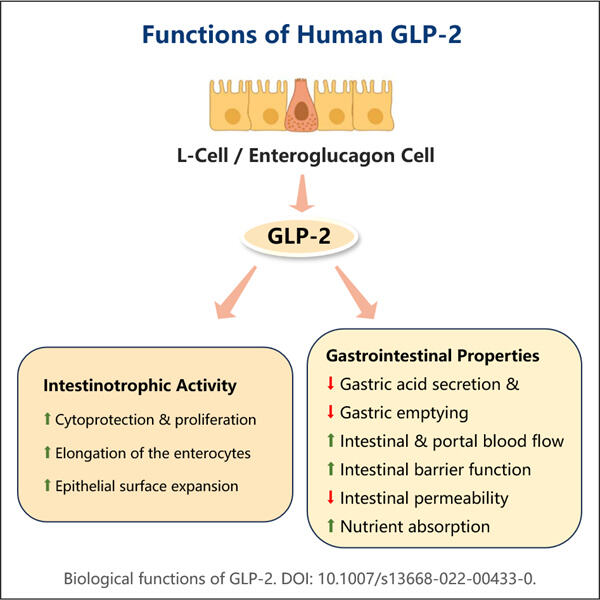
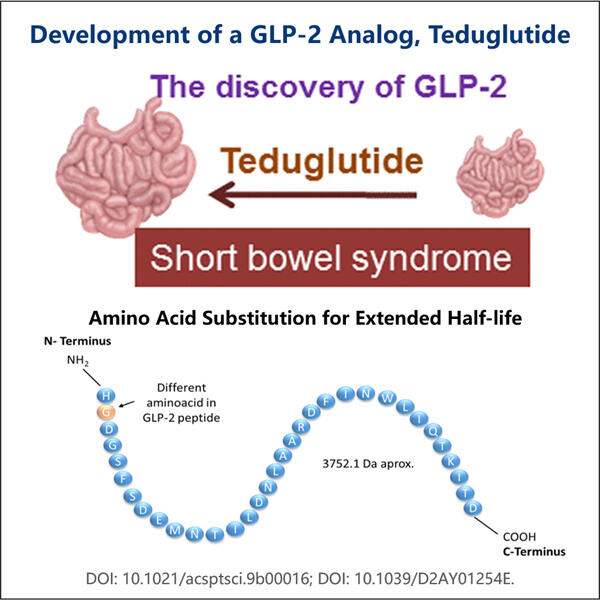
Το Yaohai Bio-Pharma, ένας ηγέτης στους CDMOs για μικροβιακά βιολογικά προϊόντα, βρίσκεται στο Τζιανγκσού. Επικεντρώνουμε την προσοχή μας στα θεραπευτικά και εμβολία που παράγονται από μικροοργανισμούς, όπως οι Αναδιπλασιαστικοί GLP-2 Αγωνιστές Βιοπαραγωγής για την υγεία του ανθρώπινου, της κτηνιατρικής και των ζώων συντροφιάς. Διαθέτουμε τις πιο προηγμένες ερευνητικές και αναπτυξιακές πλατφόρμες, καθώς και τεχνολογία παραγωγής που καλύπτουν την ολόκληρη διαδικασία παραγωγής, από την ανάπτυξη μικροβιακών φυλών, την αποθήκευση κυττάρων, την ανάπτυξη διαδικασίας και μεθόδους μέχρι την κλινική και εμπορική παραγωγή, που εγγυάται επιτυχή παραγωγή νέων λύσεων. Έχουμε αποκτήσει μεγάλη εμπειρία στη βιοεπεξεργασία μικροβιακών κυττάρων. Πάνω από 200 έργα έχουν ολοκληρωθεί με επιτυχία, και υποστηρίζουμε τους πελάτες μας να περάσουν από τις κανονιστικές απαιτήσεις, όπως εκείνες της US FDA και της EU EMA. Επίσης, τους βοηθούμε με την Australia TGA και την China NMPA. Η εμπειρία και η ειδικευμένη γνώση μας, καθώς και η εκτεταμένη γνώση μας, μας επιτρέπει να ανταποκριθούμε γρήγορα στις απαιτήσεις της αγοράς και να προσφέρουμε προσαρμοσμένες υπηρεσίες CDMO.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN