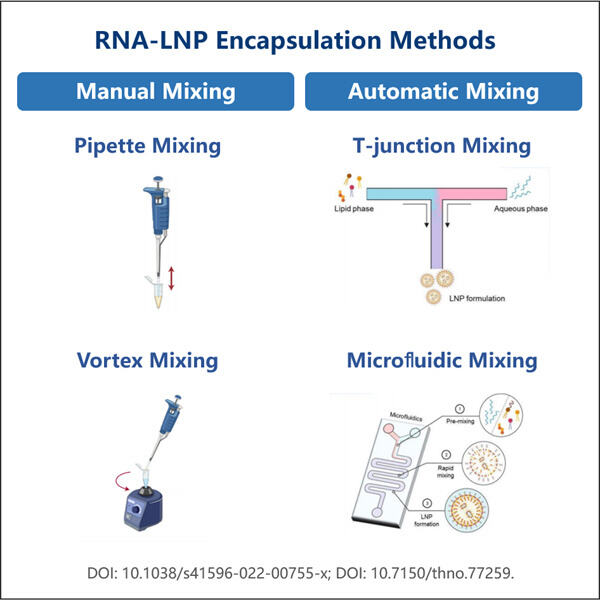
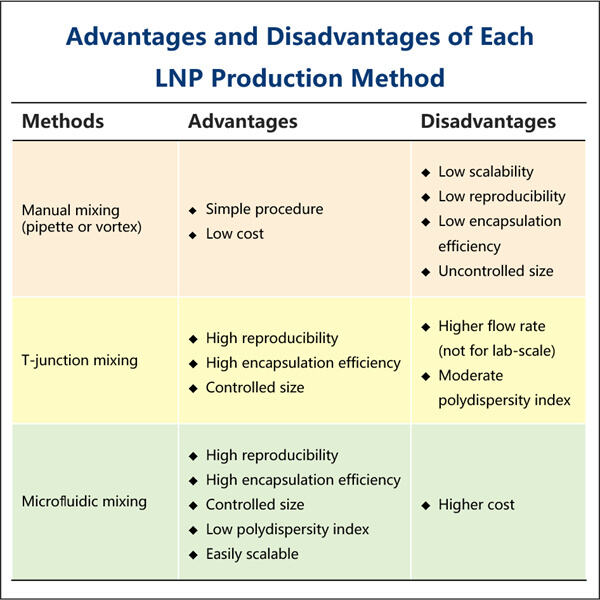
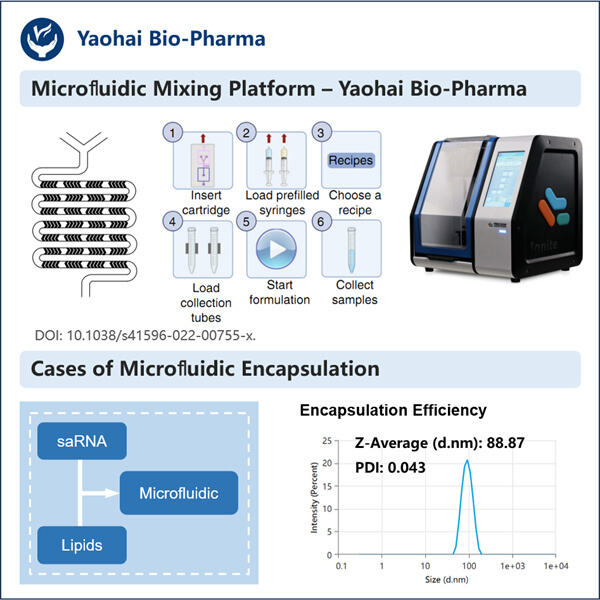
Then comes Yaohai; with the saRNA-LNP Encapsulation Protocol (a method to prepare RNA-polyplexes). So what this discovery is in fact, a new scientific theory that could be applied to so many different disease states that we manage in people today. In this protocol, we will discuss more on this and who all may be beneficial.
saRNA-LNP Encapsulation Protocol… you can see from the middle of the list how this is another way (but not direct) to get mRNA inside our cells. The mRNA is then encapsulated in a tiny lipid nanoparticle (LNP). These are special molecules which wrap around the mRNA to shield it from break-down and for it to be better before being ripped in shreds on its way through the body, of getting into the cells where you want them.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN