Personalizare, eficiență și rentabilitate
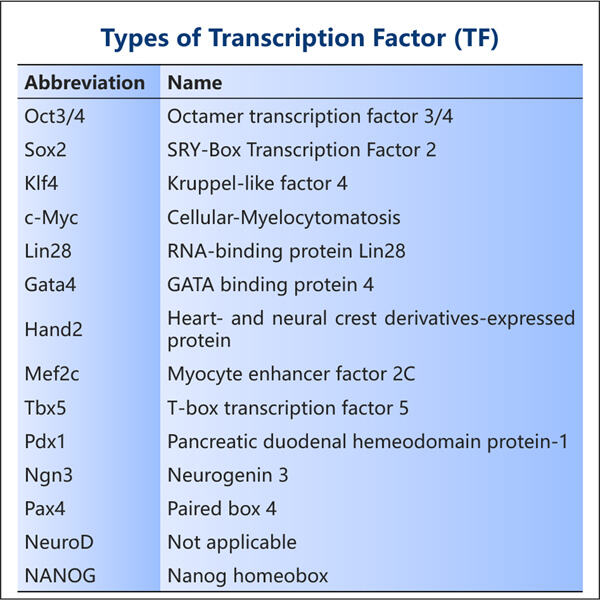
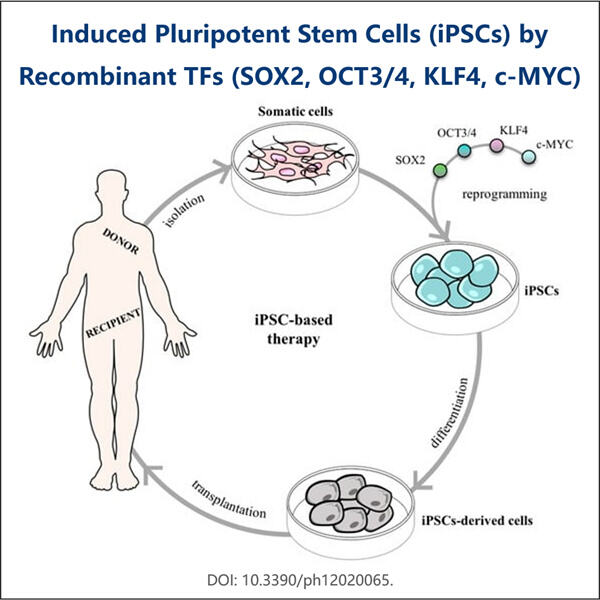
Yaohai Bio-Pharma are experiență în fabricarea de substanțe biologice create din microorganisme. Oferim soluții RD personalizate, precum și servicii de producție, minimizând riscul. Am lucrat cu diverse modalități, cum ar fi vaccinuri subunități recombinante, peptide, hormoni, citokine, factori de creștere, anticorpi mono-domenii, enzime, plasmid, ADN, ARNm și alte microorganisme extracelulare și secrete intracelulare. (produce până la 15 g/L) bacterii intracelulare solubile și corpi de incluziune (randament până la 10 g/L) Am creat, de asemenea, un sistem de fermentație BSL-2 pentru a crea vaccinuri cu factor de transcripție recombinant. piata

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NU
NU
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN