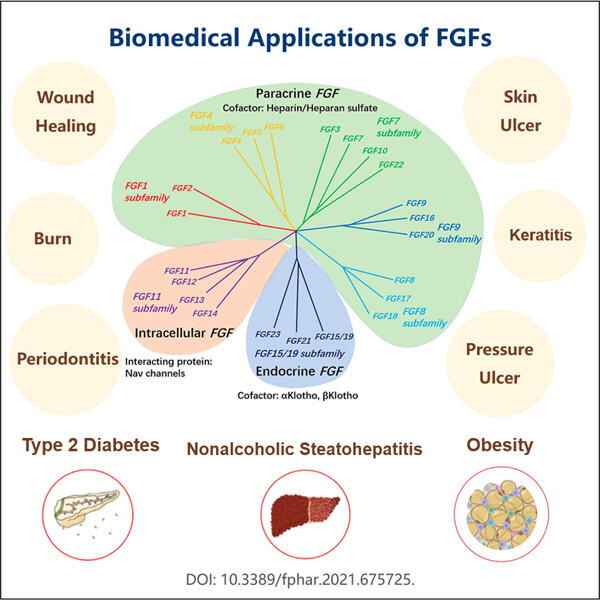
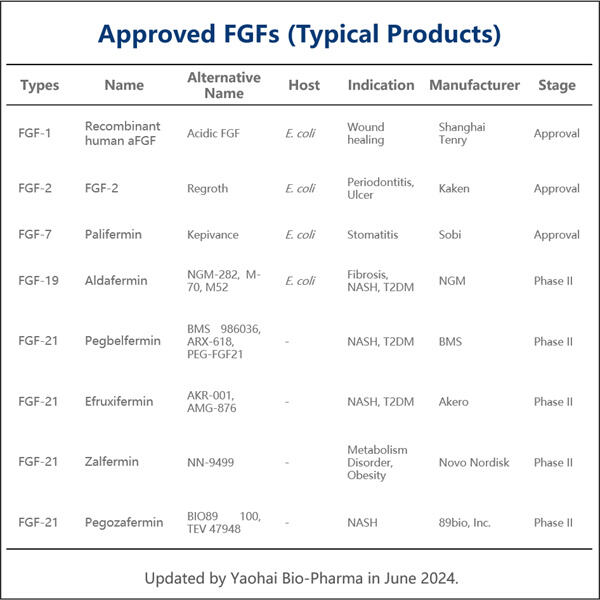
Yaohai: A company that produces medicine for the sick. Recombinant FGF is a vital medicine they produce. FGF is a specific type of protein that is important for recovery of healing in your body. Yaohai is constantly looking for better ways to create medicine that can be even more helpful to people. They are committed to advancing the health of human beings through technology.
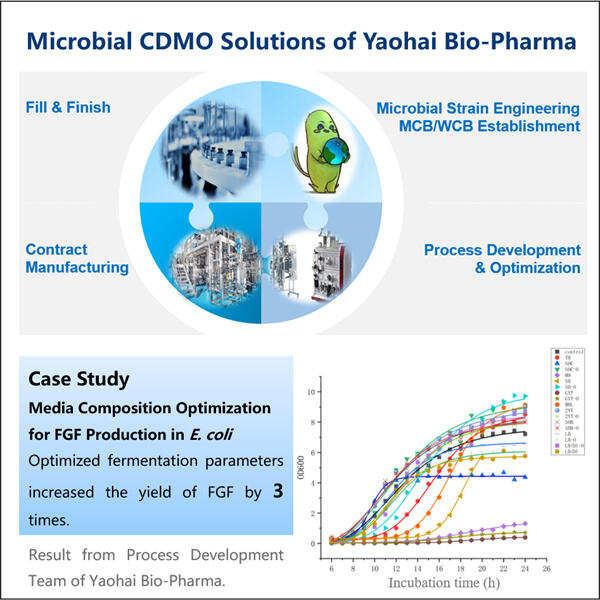
In Yaohai, the scientists laboriously create Recombinant FGF in the laboratory. In this lab, they are using special cells — they act like little factories. These cells make the FGF. The Yaohai scientists have a new way to boost the levels of FGF that these cells make. They use a special machine, called a bioreactor, to help the cells grow and produce even more FGF. “This machine provides the ideal setup to grow the cells and make the all-important protein for healing.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NU
NU
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN