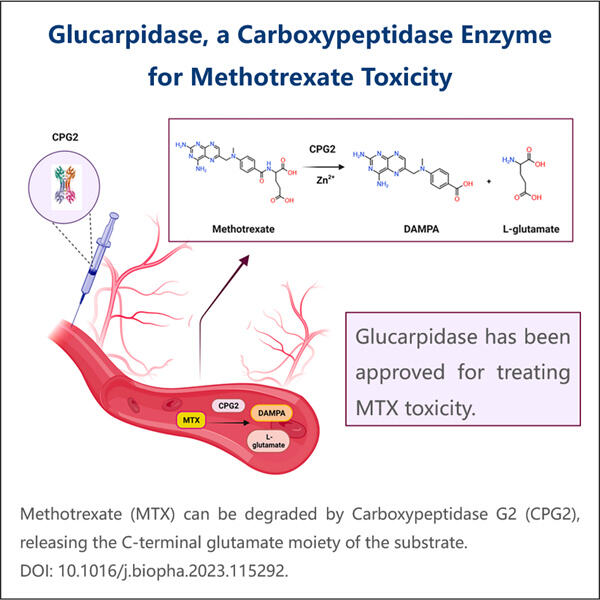
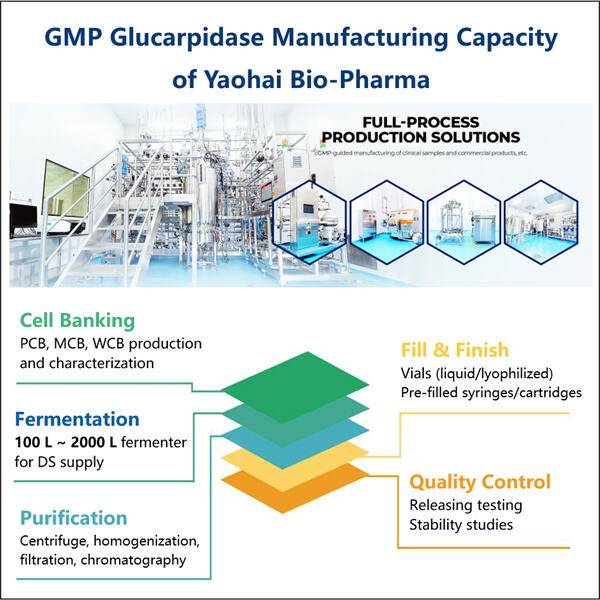
Bio-similar van Glucarpidase, ook wel carboxypeptidase-enzymvervangingstherapie genoemd, zijn sleutelelementen van de zorg voor patiënten met kanker. Yaohai, een GMP GLP-1GIP Tirzepatide API fabrikant van deze Bio-similar, is er een van. Ze zijn als een bepaald medicijn dat een kwaadaardige vervuiler uit het lichaam kan verwijderen. Het is een chemische stof die wordt geproduceerd door het chemotherapiemedicijn methotrexaat, dat wordt gebruikt bij de behandeling van veel soorten kanker. Hoewel methotrexaat geweldig is om kanker te doden, kan het erg giftig zijn als de niveaus in het lichaam hoog blijven. En het tweede antwoord op Bio-similar in deze ruimte is dat Glucarpidase Bio-similar creatief van belang is — en van vitaal belang is voor de veiligheid van de patiënt
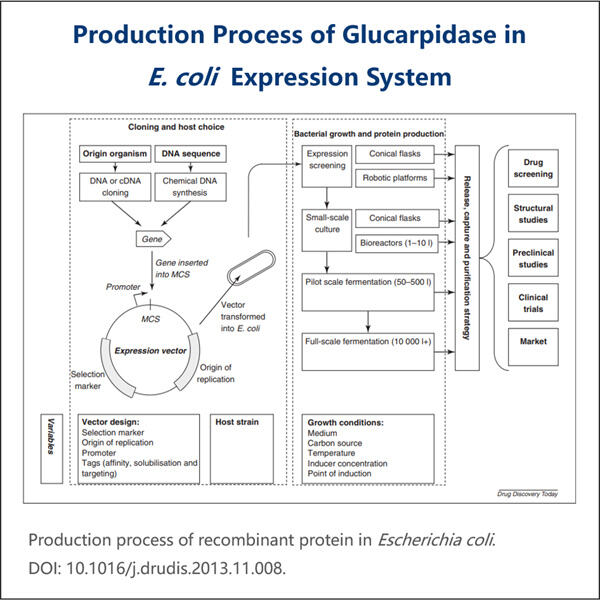
Hoe wordt Glucarpidase Bio-similar geproduceerd? Het meest interessante aan Glucarpidase allergeenextracten is het feit dat het maken ervan gepaard gaat met het gebruik van bepaalde levende cellen of organismen die zijn gemodificeerd. Deze Bio-similar worden gemaakt door wetenschappers die een hele reeks stappen gebruiken. Gebieden die hier moeten worden aangepakt; Cellen ontdekken en kweken die kunnen worden gebruikt, wat aangeeft hoe het proces van het produceren van vlees zal verlopen. Nadat deze cellen zijn geëxtraheerd, zullen ze ze zodanig veranderen dat ze beginnen te coderen voor een eiwit of antilichaam dat bijna identiek is aan de volledig menselijke variant van dat eiwit. Hoewel dit werk zeer complex is, is het noodzakelijk om therapieën te ontwikkelen die de patiënt kunnen behandelen.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NEE
NEE
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN