Pasokan Rantai & Kemampuan Manufaktur yang Kuat
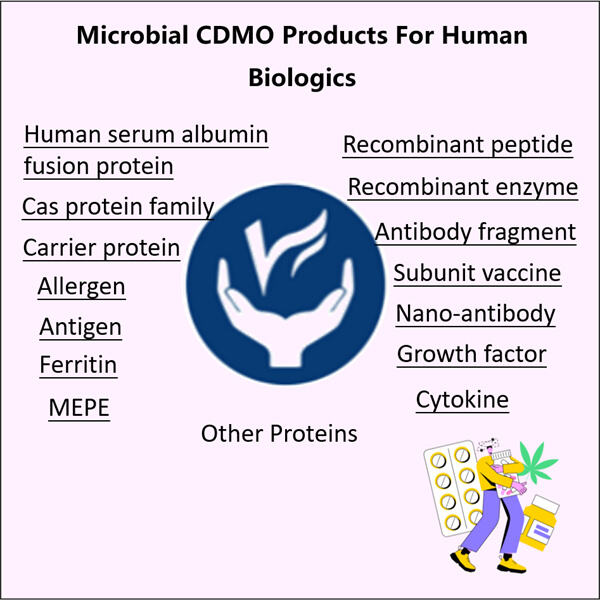
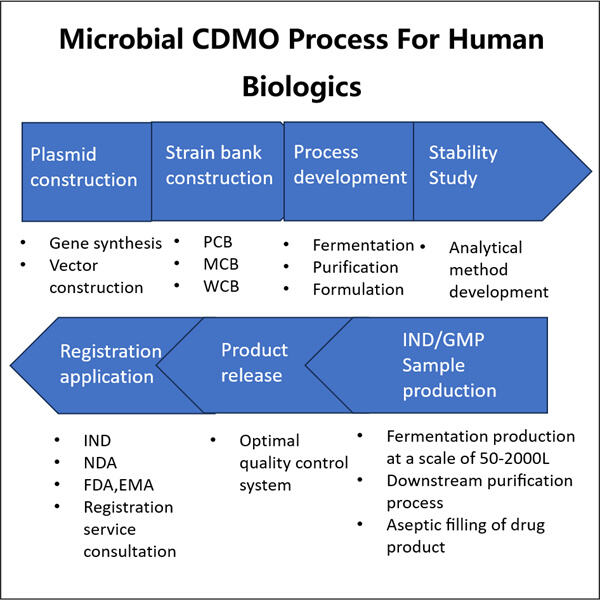
Yaohai Bio-Pharma, salah satu produsen biologis teratas 10, khusus dalam fermentasi mikroba. Kami telah menciptakan fasilitas Microbial CDMO untuk produksi Biologis Manusia dengan kemampuan RD yang kuat dan fasilitas manufaktur terdepan. Lima jalur produksi zat obat sesuai standar GMP untuk memurnikan dan mengfermentasi sel mikroba, serta dua jalur pengisian untuk botol kaca dan kartu, serta jarum yang sudah diisi sebelumnya, tersedia. Skala fermentasi yang tersedia meliputi 100L, 500L, 1000L, dan 2000L. Spesifikasi pengisian botol berkisar antara 1ml - 25ml. Spesifikasi pengisian kartrid atau suntikan yang sudah diisi berkisar antara 1-3ml. Bengkel produksi mematuhi cGMP dan menyediakan pasokan stabil produk komersial dan sampel klinis. Fasilitas kami memproduksi molekul besar yang dikirimkan ke seluruh dunia.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN