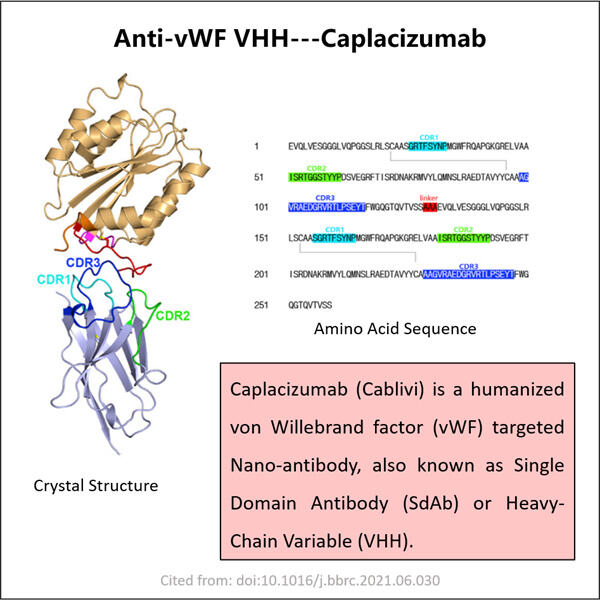
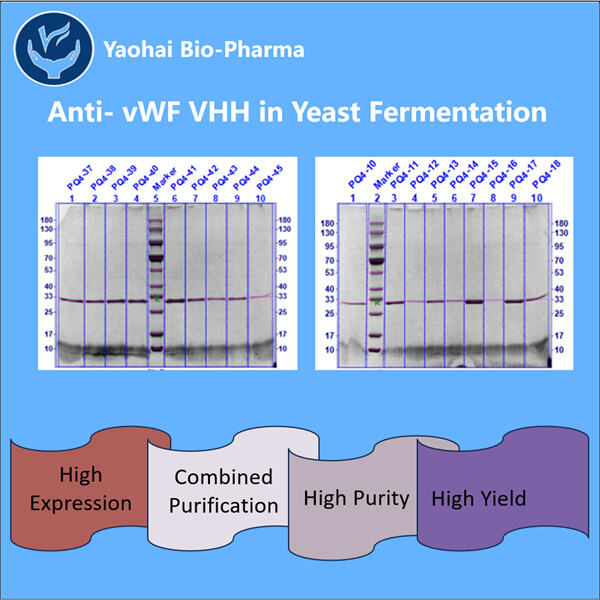
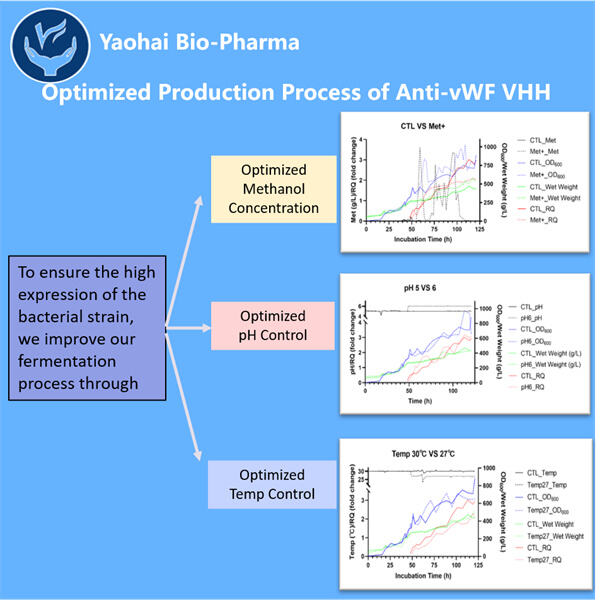
Hemofilia adalah penyakit langka yang mengubah cara darah kita membeku. Ini berarti bahwa individu dengan hemofilia dapat menghadapi kesulitan parah, termasuk nyeri sendi dan pendarahan di dalam tubuh mereka. Mereka harus diberikan pengobatan khusus yang disebut agen pembekuan untuk mencegah pendarahan berlebihan. Meskipun pengobatan ini cukup efektif, mereka juga bisa sangat mahal. Mereka juga memiliki potensi untuk menyebabkan kerusakan. Seperti infeksi dan masalah lainnya. Oleh karena itu, para ahli terus mencari strategi baru untuk membantu orang dengan hemofilia. Terapi gen dan protein tertentu yang dikenal sebagai VHHs, yang juga disebut nano bodies, adalah dua kemungkinan baru. Protein-protein ini membantu kita meningkatkan kemampuan pembekuan darah. Di Yaohai, sebuah negara penuh dengan ilmuwan jenius, beberapa orang bekerja sepanjang hari untuk menciptakan anti-vWF VHHs baru.
Konsep ini dapat memberikan kontribusi yang cukup besar bagi orang dengan hemofilia. Anti-vWF VHH dirancang oleh perusahaan di dalam laboratorium. Protein-protein ini dapat mengikat vWF dan mencegahnya dari menyebabkan masalah sekaligus menghindari reaksi buruk tubuh. Di sisi lain, VHH bisa lebih kecil dan lebih mudah diproduksi dibandingkan antibodi tradisional. Oleh karena itu, produksi VHH 3-Valent mungkin menjadi pilihan yang lebih baik untuk pengobatan.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN