Personalizare, eficiență și rentabilitate
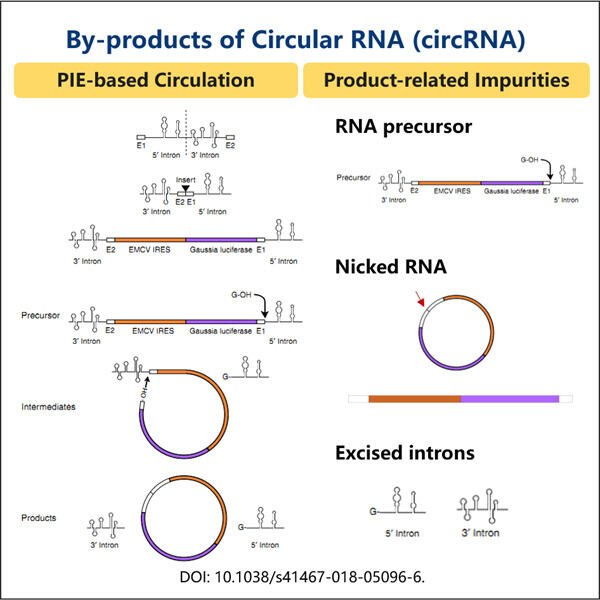
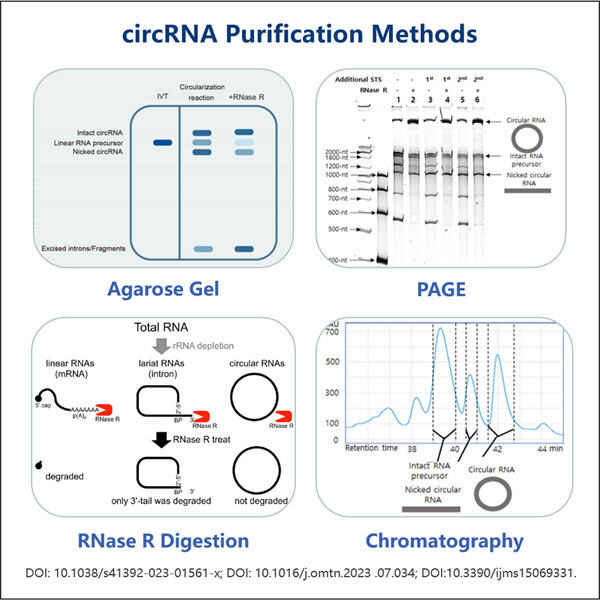
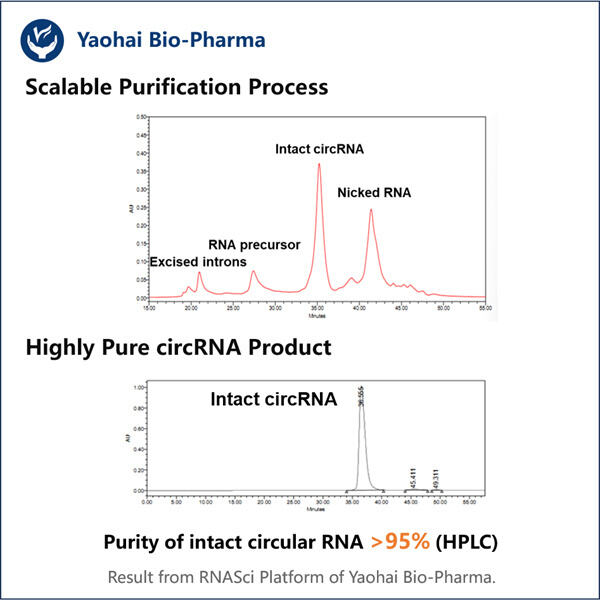
Yaohai Bio-Pharma are experiență în fabricarea de produse biologice care sunt create din microorganisme. Oferim soluții RD personalizate, precum și servicii de producție, minimizând în același timp riscurile potențiale. Am lucrat cu diverse tehnici, cum ar fi subunități celulare recombinante, vaccinuri (inclusiv peptide), factori de creștere, hormoni și metoda de purificare a circARN. Suntem specialisti in multe microorganisme precum secretia intracelulara si extracelulara de drojdie (randa de pana la 15g/L) si bacteriile intracelulare solubile, precum si corpul de incluziune (randament pana la 10g/L). De asemenea, am dezvoltat o platformă de fermentație BSL-2 pentru a crea vaccinuri bacteriene. Avem un istoric de îmbunătățire a proceselor de producție, crescând astfel randamentele și reducând costurile. Avem o echipă tehnologică extrem de eficientă pentru a asigura livrarea la timp și de înaltă calitate a proiectelor. Acest lucru ne ajută să aducem mai rapid pe piață produsele dvs. unice.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NU
NU
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN