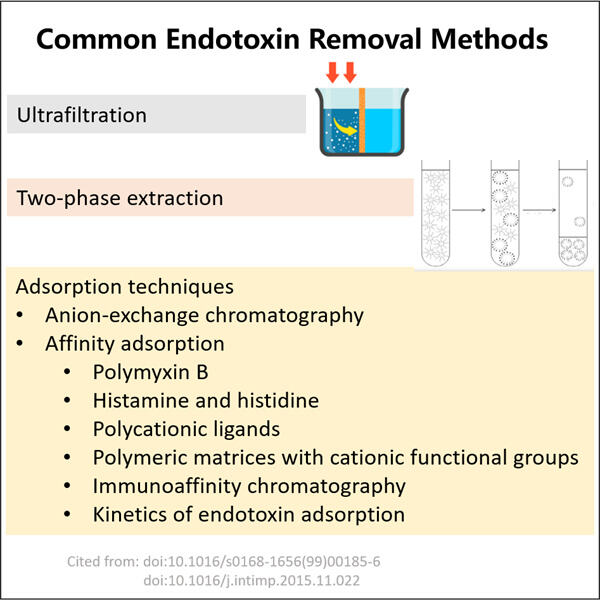
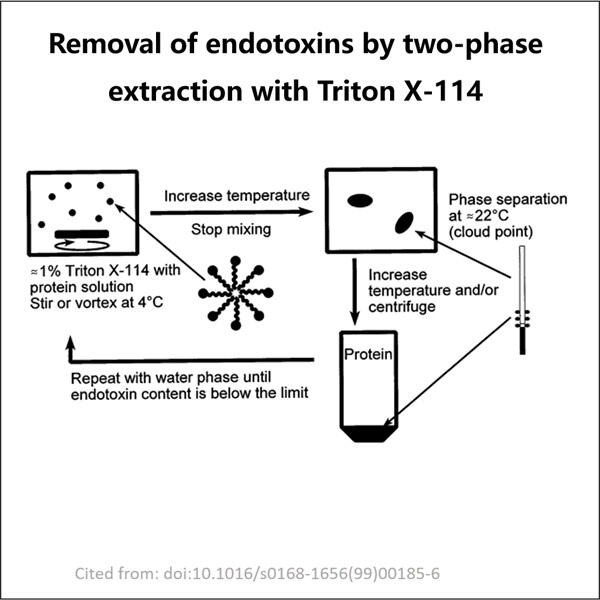
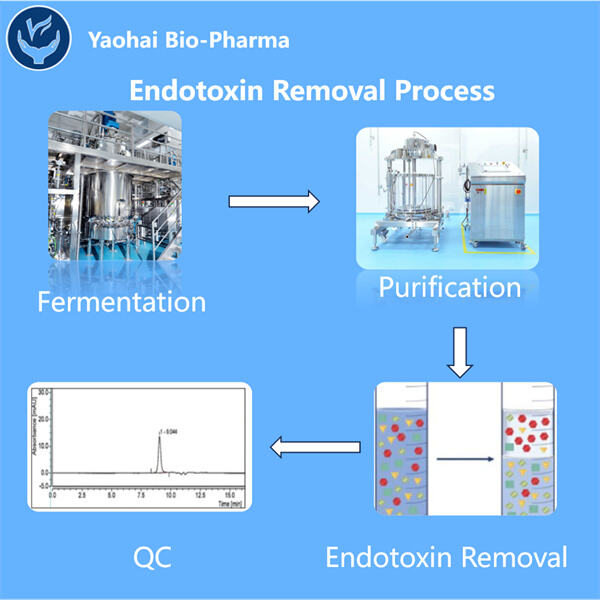
Endotoxins are minuscule molecules found in numerous forms of existing life, such as your body and those of animals. They are teeny enough invisible to the human eye but can do so much damage. Endotoxins, when not eliminated properly from either humans or animals, is considerably bad. All things considered, this is why eliminating endotoxins is imperative. It is a life and death situation that should be handled with respect.
In order to explain how we eliminate endotoxins, it is important that you know what these are. Endotoxins are a unique class of molecules that are made by some bacteria. These bacteria are ubiquitous, and can be found in soil, water and even inside our bodies. As these bacteria die, they release endotoxins into their immediate environment.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 TIDAK
TIDAK
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN