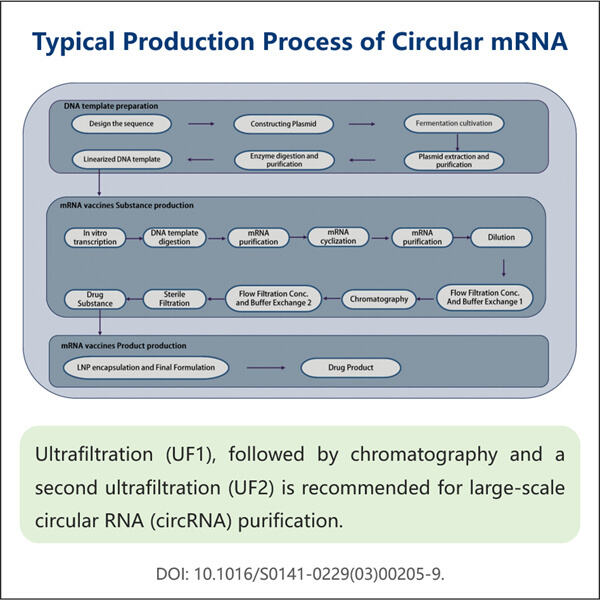
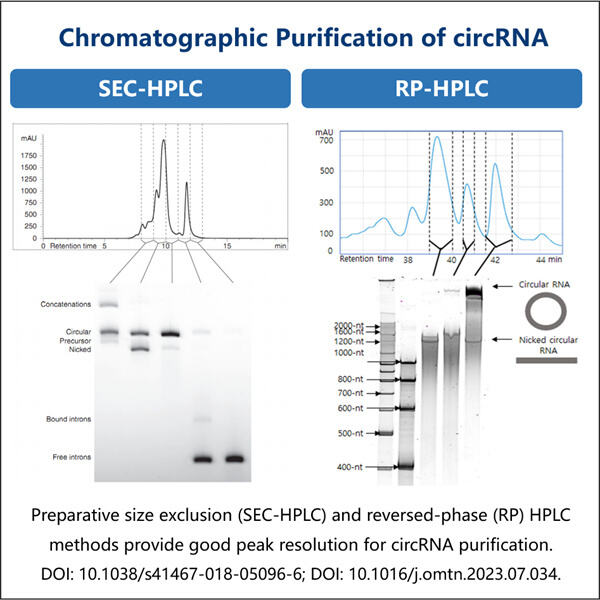
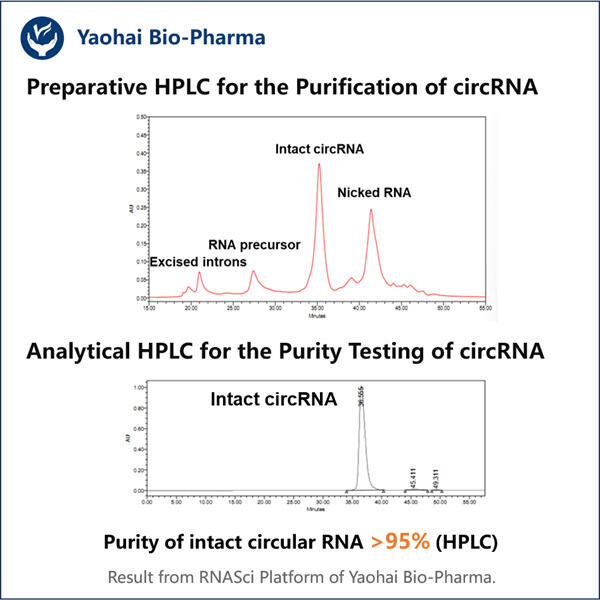
Researchers are looking into something called circRNA Due to this circular nature a new kind of RNA it created, now RNA can be in the form or circle rather than statraight line(the conventional form of most RNAs). CircRNA is expected to play a significant role in the medical and biological fields. Nevertheless, the problem and a big challenge for them are to obtain clean and enough quantities of circRNA. I have removed it once in my Username and Semicolon blog so below is the command I used to delete it however that was not as easy as it sounds because it requires proper techniques or way. Which is where Yaohai, and its clever new method for purifying circRNA, comes in.
However, in the lab of highprofile ECS author Yaohai has developed a new method to make circRNA more ideally and more cleanly. This is interesting because it reveals what governs circRNA quality while we extract them. There are many things that can effect the quality of how good the circRNA is, such as extraction sample type, amount of rna in this sample, the contamination by other substances etc. Yaohai with this new filter is able to enrich circRNA and free it from impurities in 15 minutes, which can significantly reduce the potential interference factors of circRNA research in basic life sciences and clinic.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN