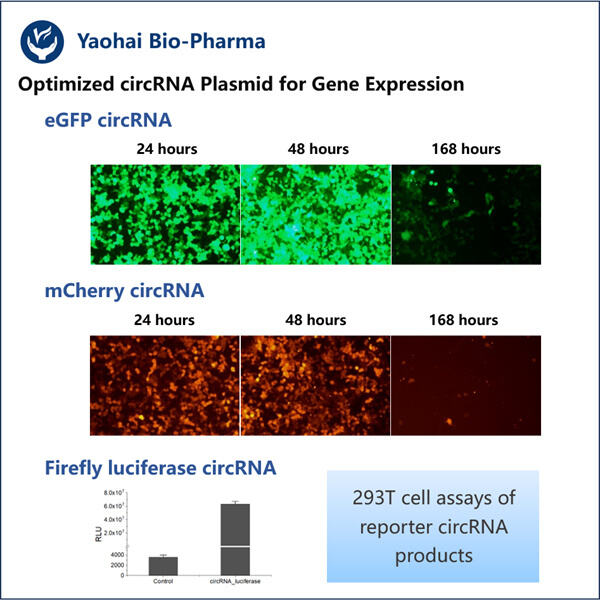
Qu Xin Li -circRNA These genes are a form of RNA that is looped into a particular circle. And unlike other forms of RNA, CIRCA has a profound impact on how the genes it guides becomes activated. They essentially serve as genes on-off switches and are implicated in a range of diseases, some of the nastiest ones out there (such as cancer).
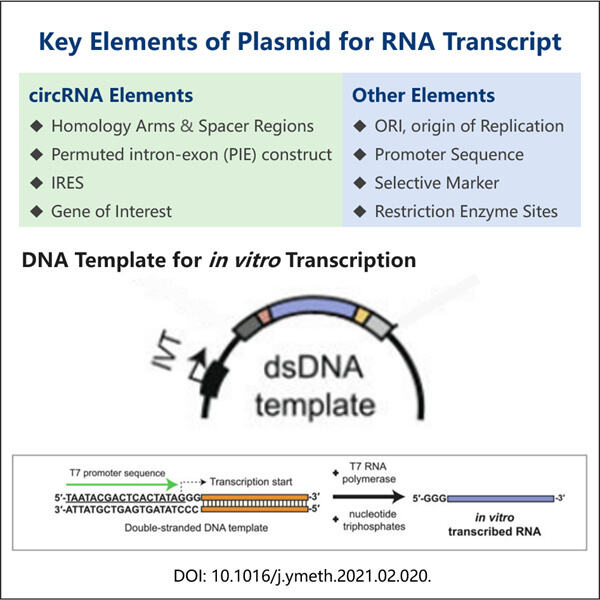
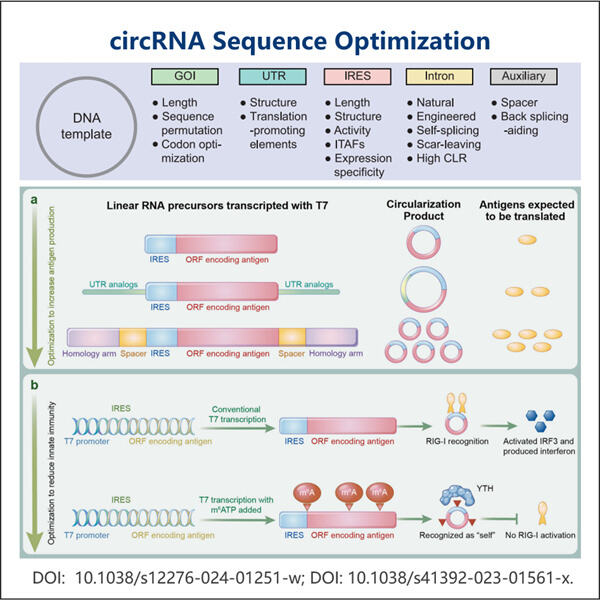
But hey, plasmids. Let's talk about them! A plasmid is a small circular piece of DNA which replications independently on its bacterial host cell. Plasmids Plasmids are small DNA molecules that can replicate independently of the chromosomal DNA and they have an important role in cloning a particular gene of interest into a vector (a carrier) for the production of multiple copies of that gene because plasmid-based vectors contain specific elements that allow them to replicate in E. coli host cells. Inside the cell, they learn from and modify DNA in order to understand how it functions. When it comes to circRNA plasmid design, scientists prepare plasmids which contain the genes encodes with circRNAs. Where that DNA is in the plasmid and how you've arranged it matters because it can impact the efficiency of circRNA gene to exert its function within the cell.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN