Combined with the requirements of mRNA quality analysis guidelines, YAOHAI has developed specifications for circRNA drug products and can provide quality analysis services for cyclic and linearized plasmid templates, circRNA drug substance and finished product of circRNA-LNP, and the service details are shown below:
Services Details
| Process |
Optional Service |
Service Details |
Delivery Period (workday) |
| Quality Control of Plasmid DNA |
Concentration/purity |
Ultraviolet (UV) Spectrophotometry |
1-2 |
| Plasmid conformation |
Agarose Gel Electrophoresis (AGE) |
| Capillary Electrophoresis (CE)-Optional |
| Plasmid identify |
Restriction Enzyme Identification/AGE |
| Quality Control of circRNA |
Concentration/purity |
Ultraviolet (UV) Spectrophotometry |
- |
| Purity |
Agarose Gel Electrophoresis (AGE)/E-Gel |
0.5 |
| HPLC-optional |
1 |
| Encapsulation Efficiency |
RiboGreen Method |
1 |
| Quality Control of circRNA-LNP |
Particle Size |
Dynamic Light Scattering (DLS)
Dynamic Light Scattering (DLS)
Dynamic Light Scattering (DLS)
|
1
1
1
|
| Polydispersity Index |
| Zeta Potential |
| Cell-based Potency Assay |
Cell Transfection |
Cell plating, cell transfection |
4 |
| Target Protein Detecting |
Fluorescence observation, Western Blot/ELISA |
1-3 |
Case study
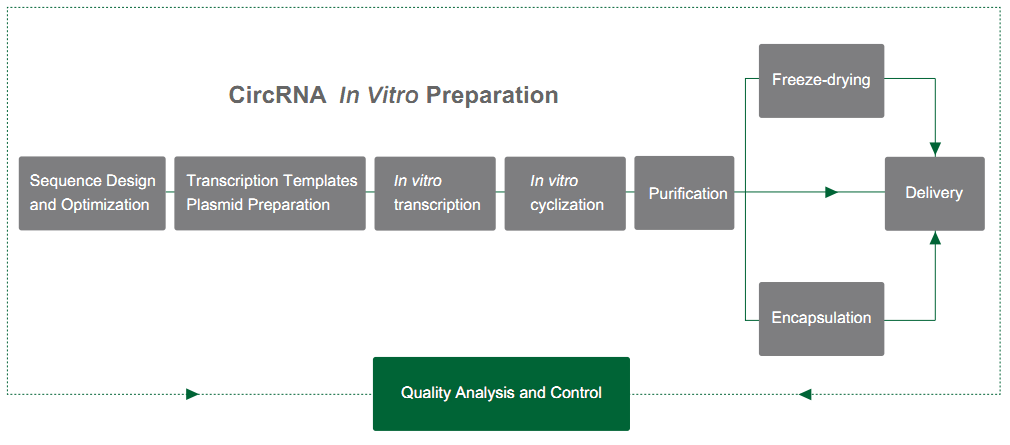
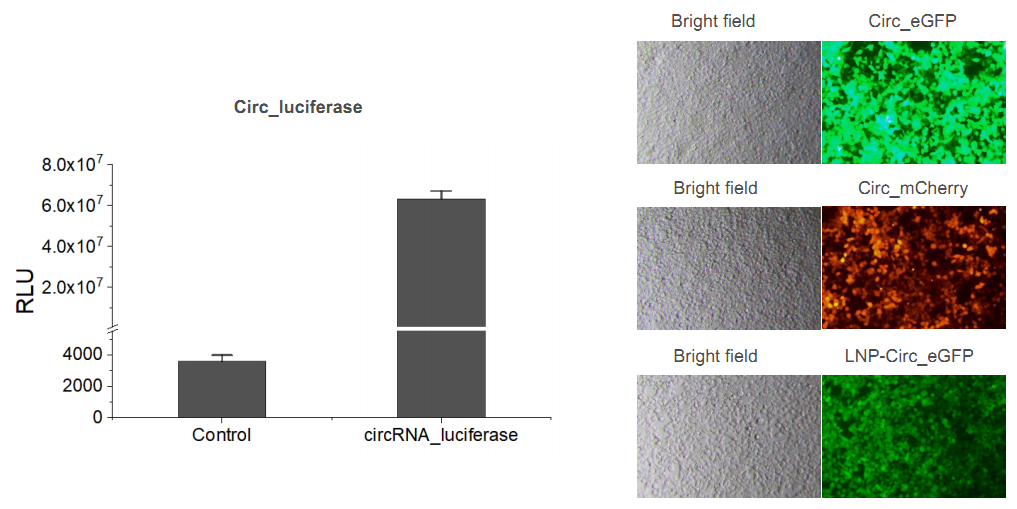
Yaohai Bio-Pharmahas built a mature platform of circRNA synthesis, purification, formulation, testing and cell transfection for circRNA drug substance and finished product of circRNA-LNP.
After transfection, the corresponding fluorescent signal, and enzyme-substrate reaction, which are taken as specific signals of the target protein can be detected.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN