According to the WHO, USP, and NMPA Guidelines for mRNA Vaccines, quality control (QC) of DNA templates, mRNA drug substance (DS) and lipid nanoparticle-mRNA (LNP-mRNA) drug product (DP) is recommended.
Yaohai Bio-Pharma can provide in-process control, batch-release solutions for circular and linearized plasmids, mRNA DS and finished LNP-mRNA to meet regulatory needs.
We design QC testing for appearance, identification, activity, purity and impurities, following ICH Quality guidelines, relevant pharmacopeia (EU and US monographs), regulatory guidelines (ICH, FDA and EMA) and GMP/GLP practices.
Abbreviation:
ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
FDA: Food and Drug Administration
EMA: European Medicines Agency
GMP: Good Manufacturing Practice
GLP: Good Laboratory Practice
Service Details
Quality Control of Template Plasmid
|
Category
|
Quality Attributes
|
Analytical Techniques
|
Research Grade
|
Clinical Supply
|
|
Physicochemical Property
|
Appearance, Visible Foreign Material
|
Visual
|
√
|
√
|
|
pH
|
Potential
|
√
|
√
|
|
Biochemical Property
|
DNA Concentration
|
UV/A260
|
√
|
√
|
|
Identity
|
Target Gene Sequencing
|
Sequencing
(Third Party)
|
--
|
√
|
|
Restriction Enzymes Digestion
|
Agarose Gel Electrophoresis (AGE)
|
√
|
√
|
|
Product-related Impurities
|
Superhelix Plasmid Purity or Linear Plasmid Purity
|
AGE
|
√
|
√
|
|
High-performance Liquid Chromatography (HPLC)
|
--
|
√
|
|
Capillary Electrophoresis (CE)
|
--
|
√
|
|
Process-related Impurities
|
Residual Endotoxin
|
Gel Method
|
--
|
√
|
|
Chromogenic Method
|
√
|
--
|
|
Host Cell Protein, HCP
|
Enzyme-Linked Immunosorbent Assay (ELISA)
|
--
|
√
|
|
Host Cell DNA -HCD
|
Quantitative Polymerase Chain Reaction (qPCR)
|
--
|
√
|
|
Host RNA
|
Reverse Transcription-Quantitative Polymerase Chain Reaction(RT-qPCR)
|
--
|
√
|
|
Residual Antibiotic
|
ELISA
|
--
|
√
|
|
Bioburden
|
Bioburden
|
Plate Counting, Membrane Filtration
|
--
|
√
|
|
Sterility
|
Direct Inoculation, Membrane Filtration
|
--
|
√
|
|
"√":Recommended,"--": Optional
|
Quality Control of mRNA
|
Category
|
Quality Attributes
|
Analytical Techniques
|
Research grade
|
Clinical supply
|
|
Biochemical Property
|
mRNA Concentration
|
UV/A260
|
√
|
√
|
|
mRNA Purity
|
A260/A280
|
√
|
√
|
|
Identity
|
mRNA Sequencing
|
Sequencing
(Third Party)
|
--
|
√
|
|
Structure Integrity
|
mRNA Integrity
|
CE
|
--
|
√
|
|
Capillary Gel Electrophoresis with Laser-Induced Fluorescence Detector(CGE-LIF)
|
--
|
√
|
|
AGE
|
√
|
√
|
|
mRNA Capping Efficiency
|
Liquid Chromatography-Mass Spectrometry(LC-MS)After Digestion
|
--
|
√
|
|
mRNA polyA Distribution
|
LC-MS After Digestion
|
--
|
√
|
|
Product-related Impurities
|
Aggregates
|
Size Exclusion Chromatography High Performance Liquid Chromatography(SEC-HPLC)
|
--
|
√
|
|
mRNA Fragments
|
Reverse Phase High Performance Liquid Chromatography(RP-HPLC)
|
--
|
√
|
|
dsRNA
|
ELISA
|
--
|
√
|
|
Process-related Impurities
|
Residual Endotoxin
|
Gel Method
|
√
|
√
|
|
Host Cell Protein, HCP
|
ELISA
|
--
|
√
|
|
Host Cell DNA -HCD
|
qPCR
|
--
|
√
|
|
"√":Recommended,"--": Optional
|
Quality Control of LNP
|
Category
|
Quality Attributes
|
Analytical Techniques
|
Research Grade
|
Clinical Supply
|
|
Biochemical Property
|
Encapsulation Efficiency
|
RiboGreen
|
√
|
√
|
|
Identify
|
Lipid Content
|
High Performance Liquid Chromatography with Charged Aerosol Detector(HPLC-CAD)
|
--
|
√
|
|
Physicochemical Property
|
Appearance, Visible Foreign Material
|
Visual
|
√
|
√
|
|
Insoluble Particles
|
Light Obscuration
|
√
|
√
|
|
Nanoparticle Diameter
|
Dynamic Light Scattering(DLS)
|
√
|
√
|
|
PDI, Polydispersity Index
|
DLS
|
√
|
√
|
|
Zeta Potential
|
DLS
|
√
|
√
|
|
pH
|
Potential
|
√
|
√
|
|
Osmolality
|
Freezing Point Titration
|
√
|
√
|
|
Deliverable Volume
|
Volumetric, Gravimetric
|
--
|
√
|
|
Safety
|
Residual Endotoxin
|
Gel Method
|
√
|
√
|
|
Abnormal Toxicity
|
Guinea Pigs
|
--
|
√
|
|
"√":Recommended,"--": Optional
|
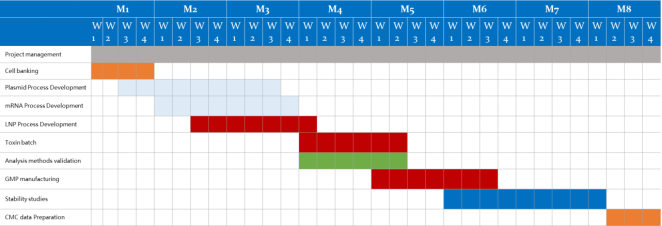
Timeline of mRNA CDMO Solutions

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN