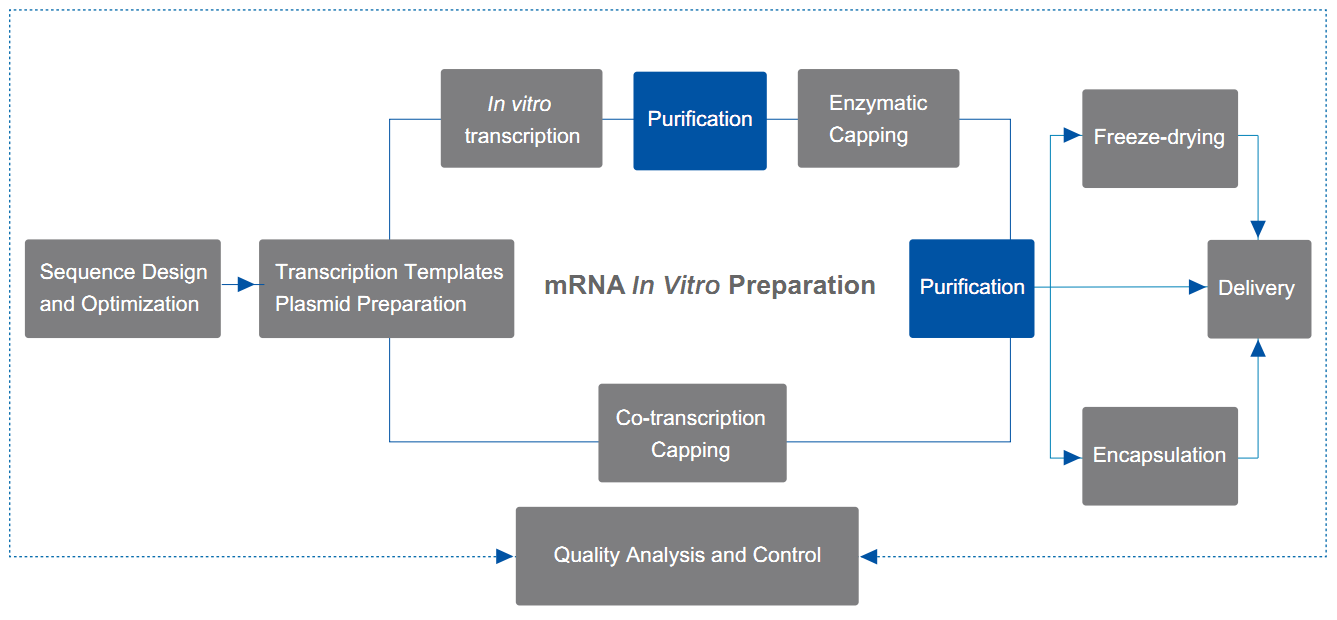
The mRNA prepared by in vitro transcription (IVT) and capping reactions requires further purification to remove unconsumed substrates and by-products during the IVT and capping reactions to ensure the efficacy and safety of mRNA vaccines or therapeutics.
Yaohai Bio-Pharma can provide mature solutions for LiCl precipitation, magnetic bead purification and chromatography purification, which can effectively remove process- or product-related impurities and prepare pure mRNA.
- LiCl precipitation method
Simple purification scheme for small amounts of mRNA, which can be used for cell transfection and some animal experiments; used for the purification of pre-capped samples after in vitro transcription.
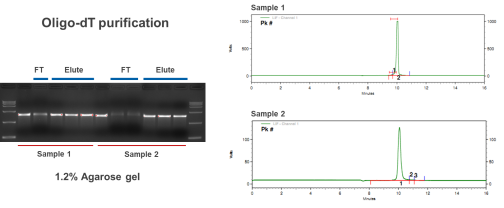
- Oligo dT magnetic bead purification method
Purification scheme for small amounts of mRNA, which can be used for cell transfection and some animal experiments; used for the purification of pre-capped samples after in vitro transcription.
- Chromatography purification method
Multiple chromatography purification solutions, such as affinity chromatography, ion exchange and hydrophobic interaction chromatography (HIC), Meet the application scenarios with higher quality requirements, such as cell transfection, LNP encapsulation, etc.
Services Details
Process |
Optional Service |
Service Details |
Delivery Period (Workday) |
Deliverable |
mRNA purification |
Conventional purification solution |
Lithium chloride precipitation |
1 |
mRNA drug substance |
| Magnetic bead purification |
| High purity purification solutions |
Affinity chromatography or multiple chromatography combinations |
2 |
| Buffer exchange |
Ultrafiltration and buffer exchange |
1 |
mRNAquality control |
Concentration measurement |
Ultraviolet spectrophotometry (UV) |
0.5 |
Test Report |
| Integrity and purity testing |
Agarose Gel Electrophoresis (AGE) |
| Capillary Electrophoresis (CE)-Optional |
1 |
Our Features
- A variety of optional purification solutions can meet different application scenarios.
- The purity of mRNA can routinely reach more than 95%, with the highest purity reaching 100%.
- Prevent mRNA degradation effectively by stringently controlling of RNase on the experimental environment and consumables.
Case study
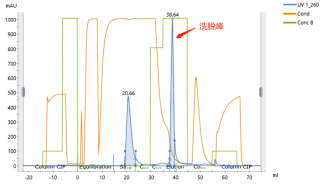
Yaohai Bio-Pharma can provide mature mRNA purification solutions that can effectively remove various process- and product-related impurities.
After capillary electrophoresis (CE) detection, the purity of the mRNA sample prepared by chromatography purification can reach more than 95%, and the residual dsRNA detected by the enzyme-linked immunosorbent assay (ELISA) kit is less than 0.06%. The high quality meets the downstream application needs of mRNA.
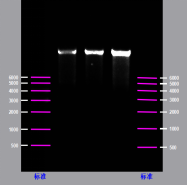
Customized Purification of mRNA (>9kb)
The purity of Spike SARS CoV-2 mRNA (more than 95%)

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN