Yaohai Bio-Pharma’s Analytical Method Development Services
Yaohai Bio-Pharma offers customized mRNA analytical development services, including the identification of plasmid, mRNA and lipid nanoparticle-mRNA (LNP-mRNA), as well as process/product-related impurities.
We provide phase-appropriate analytical method development and validation solutions. Our team has significant expertise in analytics for all stages of the biologics development lifecycle, from early-stage protocols to late-stage quality control method optimization. Tests are designed in consideration of the relevant pharmacopeia (EU and US monographs), regulatory guidelines (ICH, FDA, and EMA), and GMP/GLP practices.
Abbreviation:
ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
FDA: Food and Drug Administration
EMA: European Medicines Agency
GMP: Good Manufacturing Practice
GLP: Good Laboratory Practice
Service Details
- Method development and optimization for in-process controls, release and stability studies
- Analytical method qualification/validation
- Quality control (QC) and stability studies of research batches
- Reference standard generation and characterization
- Technology transfer to QC
Method Development and Pre-Validation:
|
Quality Attribute
|
Analytical Methods
|
|
CircularPlasmid
(circPlasmid)
|
Supercoiled Plasmid Percentage
|
Capillary Electrophoresis-Laser-Induced Fluorescence (CE-LIF)
|
|
Anion Exchange High-Performance Liquid Chromatography (AEX-HPLC)
|
|
Host Cell Protein (HCP)
|
Enzyme-Linked Immunosorbent Assay (ELISA)
|
|
Host Cell DNA (HCD)
|
Quantitative Polymerase Chain Reaction (qPCR)
|
|
Residual RNA
|
qPCR
|
|
Residual Protein
|
Bicinchoninic Acid Assay(BCA)
|
|
Residual Antibiotics
|
ELISA
|
|
LinearizedPlasmid
|
Linearized Plasmid Purity
|
Capillary Electrophoresis (CE)
|
|
mRNA
|
mRNA Integrity
|
CE, Capillary Gel Electrophoresis with Laser-Induced Fluorescence Detector (CGE-LIF)
|
|
Product Related Impurities - Aggregates
|
Size Exclusion Chromatography High Performance Liquid Chromatography (SEC-HPLC)
|
|
Product Related Impurities - Fragment mRNA
|
Reverse Phase High Performance Liquid Chromatography (RP-HPLC)
|
|
Product Related Impurities -dsRNA
|
ELISA
|
|
mRNA Capping Efficiency
|
Liquid Chromatography-Mass Spectrometry (LC-MS) After Digestion
|
|
PolyA Distribution
|
LC-MS After Digestion
|
|
Host Cell Protein (HCP)
|
ELISA
|
|
Host Cell DNA (HCD)
|
qPCR
|
|
Residual DNA
|
qPCR
|
|
LNP-mRNA
|
Lipid Component
|
High Performance Liquid Chromatography with Charged Aerosol Detector (HPLC-CAD)
|
|
Lipid Content
|
HPLC-CAD
|
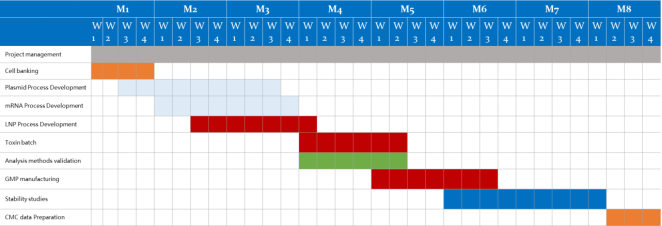
Timeline of mRNA CDMO Solutions

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN