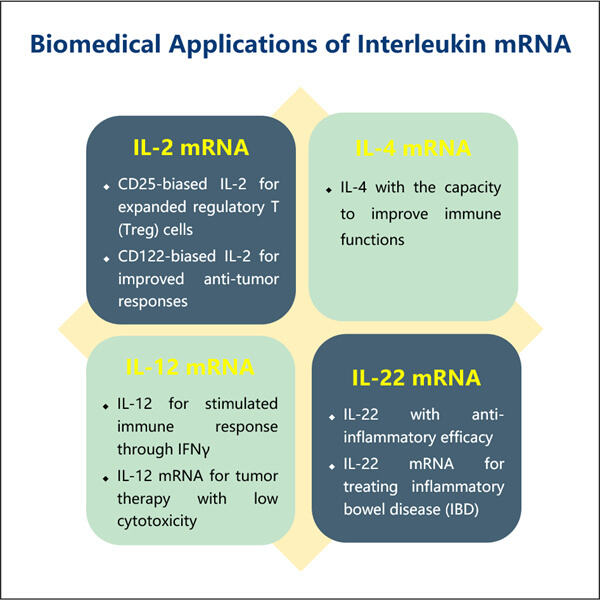
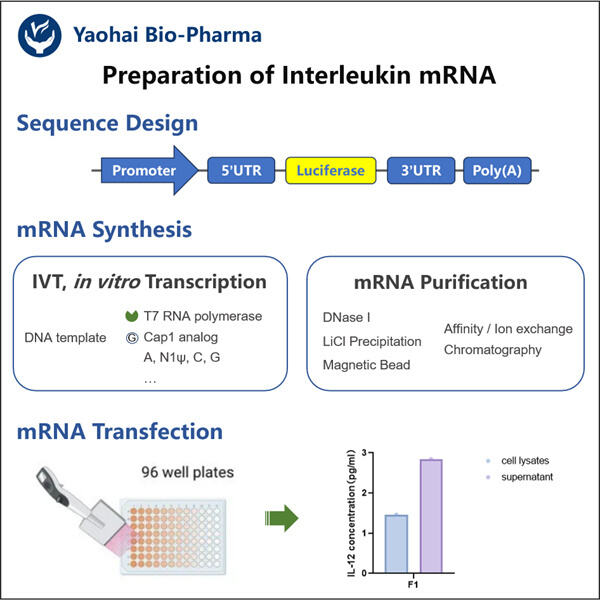
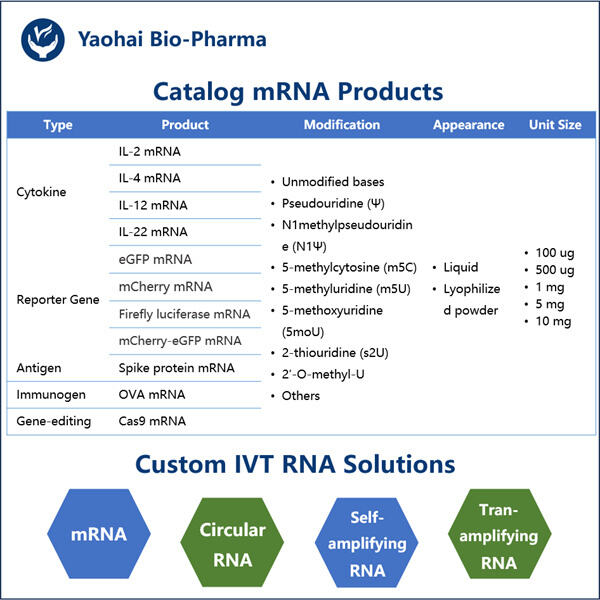
Posledice za dijagnozu i liječenje bolesti
Bolesti autoimune su esencijalno uzroke bludnim reakcijama gdje imunski sistem ne može razlikovati između normalnih, zdravih tkiva i antigena i počinje napadati ova tkiva sa glavnim primerima kao što je reumatska artritis. To, pored drugog, uzrokuje upale koje uzrokuju bol i neprijatnosti. Kao posledica, na osnovu nivoa interleukina mRNA i mnogih drugih čimbenika koje oni proveravaju u krvi, lekari mogu da merene koliko upale ide unutar tela. To im pomaga da biraju intervencije kako bi poboljšali pacijenta ili barem ih spriječili od toga da postanu lošiji.
Aktivacija vašeg imunskog sistema za produženo vrijeme takođe može uzrokovati kroničnu upalu i to može imati ozbiljne zdravstvene posledice na dugoročnom planu. To uključuje stvari poput određenih vrsta bolesti srca, dijabetesa i čak raka. Prema WebMD proizvodnja plazmida mRNA , imunski sistem izlazi iz ravnoteže i oslobađa preveliki količina cytokina — uključujući interleukin — koji zatim napada same organe i tkiva našeg tela.

 SR
SR
 EN
EN AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN