Help U Snel Begrijpen Wat CMO, CDMO, en CMO Zijn
In de farmaceutische en biotechnologie industrieën is het kiezen van de juiste partner voor medicijnontwikkeling en productie cruciaal. Hoewel Contract Research Organizations (CRO's) en Contract Manufacturing Organizations (CMO's) waardevolle diensten bieden, bieden Contract Development and Manufacturing Organizations (CDMO's) een geïntegreerdere aanpak die het hele proces kan versnellen.
CRO, CMO en CDMO begrijpen:
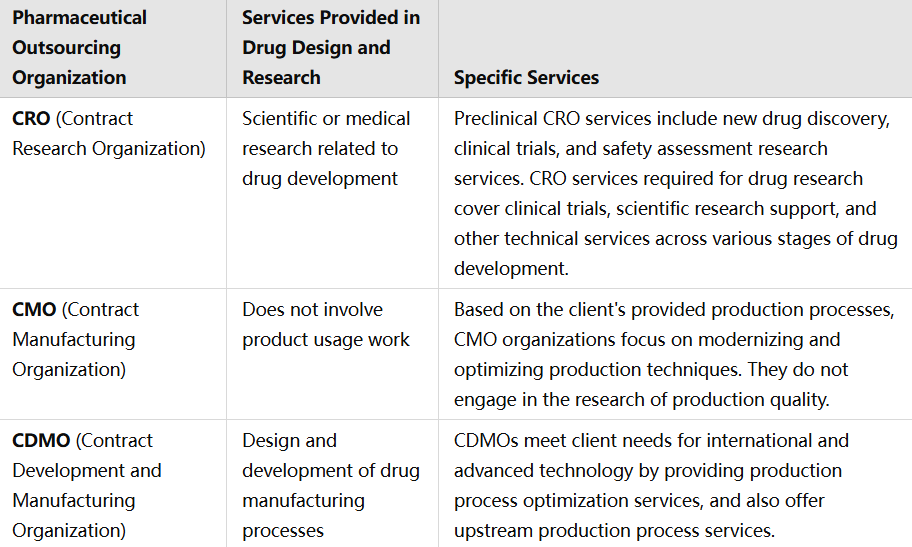
Contract Research Organizations (CRO's): CRO's specialiseren zich in het onderzoeksfase, met diensten zoals beheer van klinische trials, preclinisch onderzoek, datamanagement en regulieringszaken. Ze richten zich voornamelijk op de vroege fasen van geneesmiddelenontwikkeling.
Contract Manufacturing Organization (CMO): CMO's concentreren zich op het productieaspect, met grote schaalproductie wanneer een geneesmiddel ontwikkeld is. Hun diensten omvatten commerciële schaalproductie, verpakkingsactiviteiten en kwaliteitscontrole-tests.
Contract Development and Manufacturing Organization (CDMO): CDMO biedt volledige oplossingen, die zowel ontwikkeling als productie omvatten. Ze bieden diensten van formulatieontwikkeling en procesoptimalisatie tot de productie van klinisch proefmateriaal en commerciële productie. Deze geïntegreerde aanpak vermindert het behoefte aan meerdere partnerschappen en vereenvoudigt het ontwikkelingsproces.
Dienstverschillen in CRO, CMO, CDMO:
Relatie tussen CDMO en verkoopproducten
CDMO-diensten zijn essentieel voor de eindlevering van het product. Geneesmiddelontwikkeling is een hoogrisicoproces met hoge investeringen en lange termijn. Om kosten te verlagen en efficiëntie te verbeteren, outsourcen farmaceutische bedrijven de ontwikkeling en productie van geneesmiddelen naar CDMOs. De diensten omvatten doorgaans procesontwerp, schaalvergroting, structuurbevestiging, stabiliteitsstudies, onreinheidsanalyse en maatwerkproductie. Na afloop worden tussenproducten of API's aan klanten geleverd.
- CDMO-diensten waarborgen het succesvolle ontwikkelen van commercialiseerbare tussenproducten of API's.
- CDMO-diensten zijn essentieel om aan de specifieke behoeften van aangepaste farmaceutische producten te voldoen.
- Eindcliënten nemen deel aan kwaliteitsaudits om veiligheid, effectiviteit en kwaliteit te waarborgen.
- CDMO houden zich aan branchestandards, wat consistent service en levering waarborgt.
Met jarenlange gewijde inspanningen heeft Yaohai Bio-Pharma een leidende eenstop-CRO/CDMO/MAH-serviceplatform in de branche opgericht. Tot op heden heeft het bedrijf succesvol meer dan 200 projecten afgerond, waaronder 3 fase III-klinische studies, 4 fase II-studies, meerdere IND-en fase I-klinische studies.
Daaronder bevinden zich 7 projecten met dubbele indieningen zowel in de VS als China, en 2 die geregistreerd staan in Australië. De projecten omvatten een verscheidenheid aan mainstreambiologica en therapieindicaties, voldoende aan regulatoire indienvereisten in verschillende wereldwijde regio's.
We zoeken ook actief naar instellingen of individuen als wereldwijde partners. We bieden de meest concurrerende vergoeding in de sector. Als u vragen heeft, aarzel dan niet om contact met ons op te nemen via [email protected]
Nieuws
-
Yaohai Bio-Pharma slaagt in EU QP-audit en haalt ISO-drievoudige certificatie
2024-05-08
-
BiotechGate, Online
2024-05-13
-
2024 WORLD VACCINE CONGRESS Washington
2024-04-01
-
CPHI North America 2024
2024-05-07
-
BIO International Convention 2024
2024-06-03
-
FCE COSMETIQUE
2024-06-04
-
CPHI Milan 2024
2024-10-08

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN

