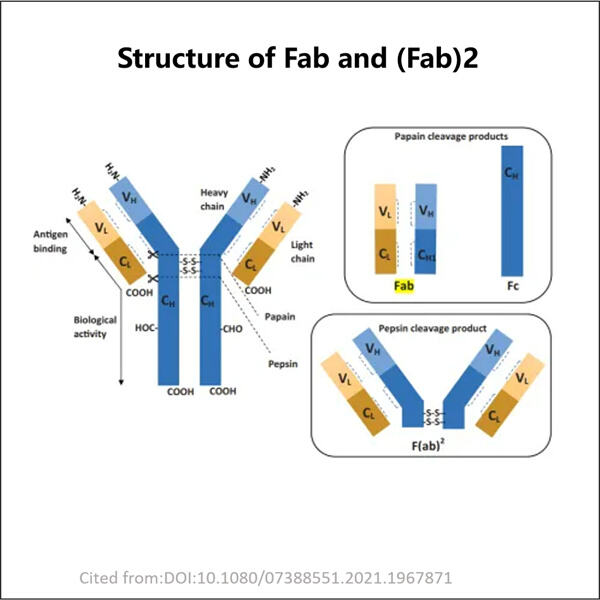
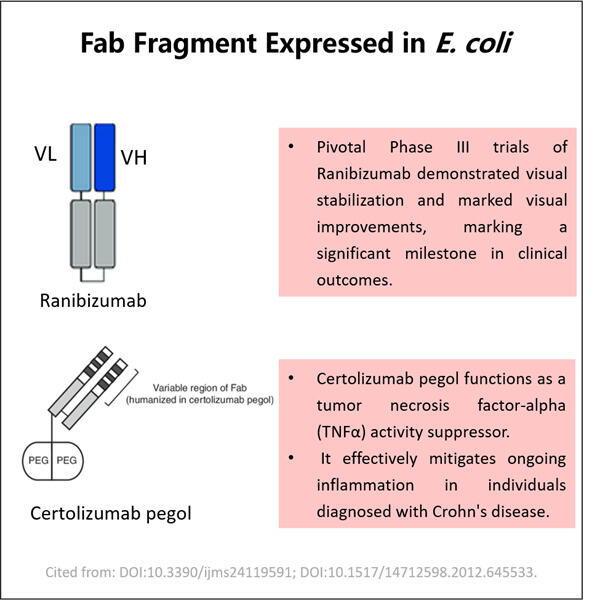
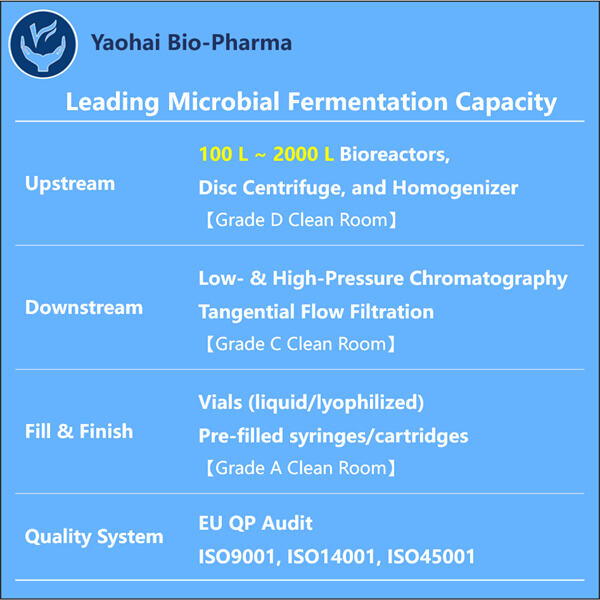
Hello, young readers. Well, today we are going to deal with something really amazing which is known by the name of Yaohai Fab Fragment Process Development. This may seem somewhat complicated, but don't worry. We Explain it for You in Simple Words Fab Fragments = a part of an antibody, Fab stands for ‘Fragment antigen-binding” For those who do not know, antibodies are crucial to our health as they fight against diseases for us. But before that, let us understand what these big words are all about.
Fab Fragment? — essentially a piece of an antibody If you like, think of antibodies as little soldiers that fight germs and the illnesses they cause. Today, we shall discuss the “GLP-1 Fragment Production". This means continuous improvement by "getting smarter" (better) at the best way to do things. And especially when we talk about Fab Fragment Process Development, it entails the fulfillment of start and intelligent generation of these small components of antibodies which are imperative in assisting the body at large scale for fighting off diseases.

 BN
BN
 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR