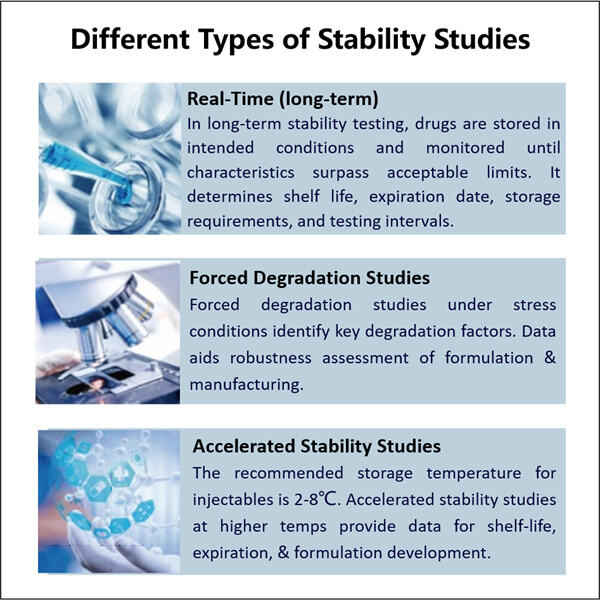
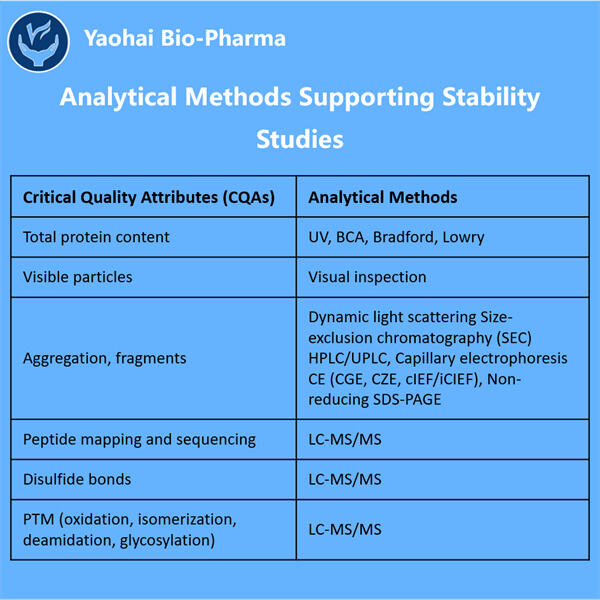
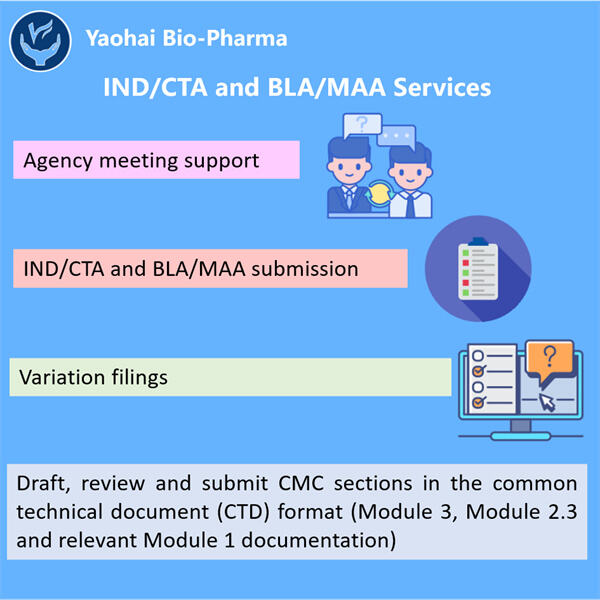
Τα Σπουδές Σταθερότητας Βιολογικών είναι εξαιρετικά κρίσιμες για τόσο: φάρμακο & την υγεία των ασθενών. Τέτοιες σπουδές επιτρέπουν σε επιστήμονες να δουν πόσο δυνατά είναι αυτά τα βιολογικά στην πραγματικότητα. Τα βιολογικά είναι ουσίες όπως πρωτεΐνες, πεπτίδια, εμβολίες και άλλα ζωντανά πράγματα που δημιουργούνται σε μια εργαστήριο. Θέλουν να μάθουν πόσο μακριά κρατούν αυτά τα προϊόντα στο ράφι, και αν είναι ασφαλή για τους ασθενείς και κάνουν ό,τι είναι αναμενόμενο να κάνουν. Χρειάζεται πολύ χρόνο και χρήματα στο Yaohai, ένα μεγάλο φαρμακευτικό εταιρεία για να κάνει αυτές τις σπουδές. Θέλουν να είναι απολύτως σίγουροι ότι τα προϊόντα τους λειτουργούν καλύτερα από ό,τι μπορούν, και να βοηθούν όταν το χρειάζονται οι άνθρωποι.
Οι Σπουδές Σταθερότητας Βιολογικών είναι σημαντικές στην παραγωγή ασφαλούς και αποτελεσματικού φαρμάκου. Αυτοί τύποι σπουδών είναι σημαντικοί για τα φαρμακευτικά βιολογικά που χρησιμοποιούνται για τη θεραπεία ενός ευρύτερου φάσματος ασθενειών, επειδή εξασφαλίζουν ότι το φάρμακο δεν είναι ευαίσθητο και χημικά σταθερό. Τα βιολογικά είναι γενικά πιο περίπλοκα από τα κανονικά φαρμακευτικά φάρμακα. Αυτά τα Yaohai Προϊόντα αποτελούνται από τεράστια μόρια, τα οποία απλώς χρειάζονται λίγο περισσότερη προσπάθεια για να τα διατηρήσουμε σε κίνηση. Αυτό είναι λόγω της μεγέθουςς και ποικιλομορφίας τους, που ξανά φτιάχνει το ότι οποιαδήποτε αλλαγή στο περιβάλλον τους επηρεάζει διαφορετικά.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN