DNA vaccines and mRNA vaccines share similarities in that both can encode any antigen related to pathogenic microorganisms or tumors, and can stimulate the immune response without the need for viral vectors or adjuvants. However, in terms of structure, DNA vaccines are more stable than mRNA vaccines. In addition to their use in infectious disease prevention, DNA vaccines have also accumulated rich clinical experience in the field of tumor therapy. DNA vaccines have a significant market application in both human and veterinary vaccine fields.
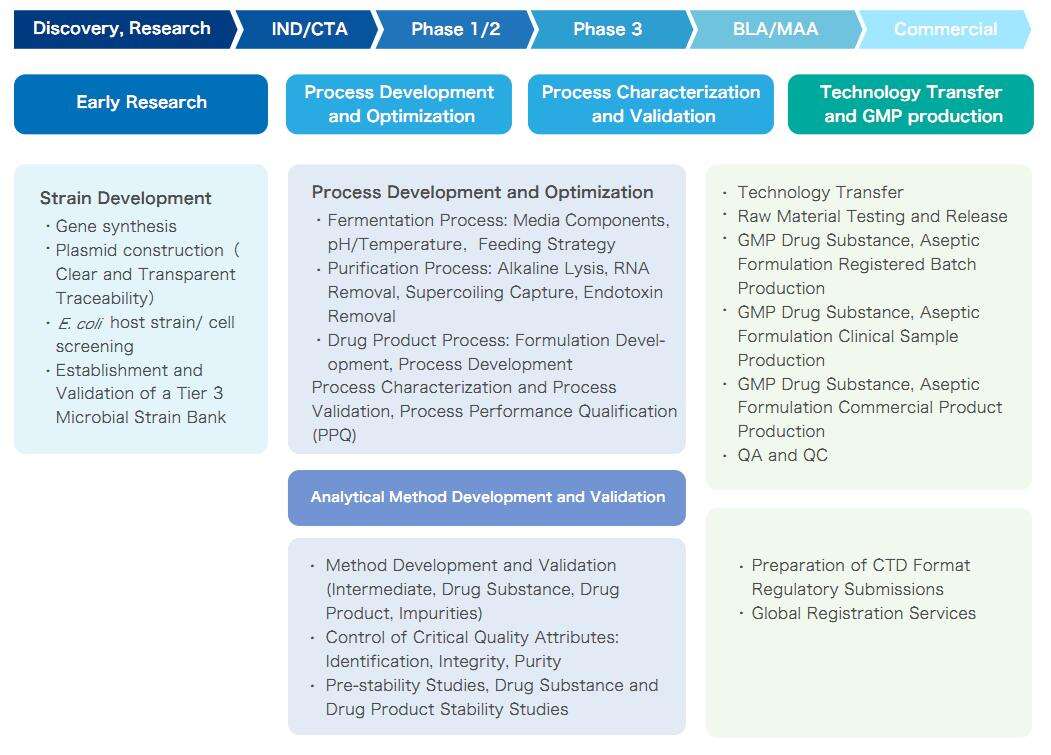
Yaohai Bio-Pharma, with its powerful process development platform and extensive experience in plasmid DNA production, can provide customers with a one-stop solution from plasmid DNA strain development to GMP production. We flexibly adjust the service process according to the customized needs of customers and provide high-quality DNA Drug Substance (DS) or Drug Product (DP) in quantities ranging from ten grams to hundreds of grams, as well as complete development and GMP production records and testing reports.
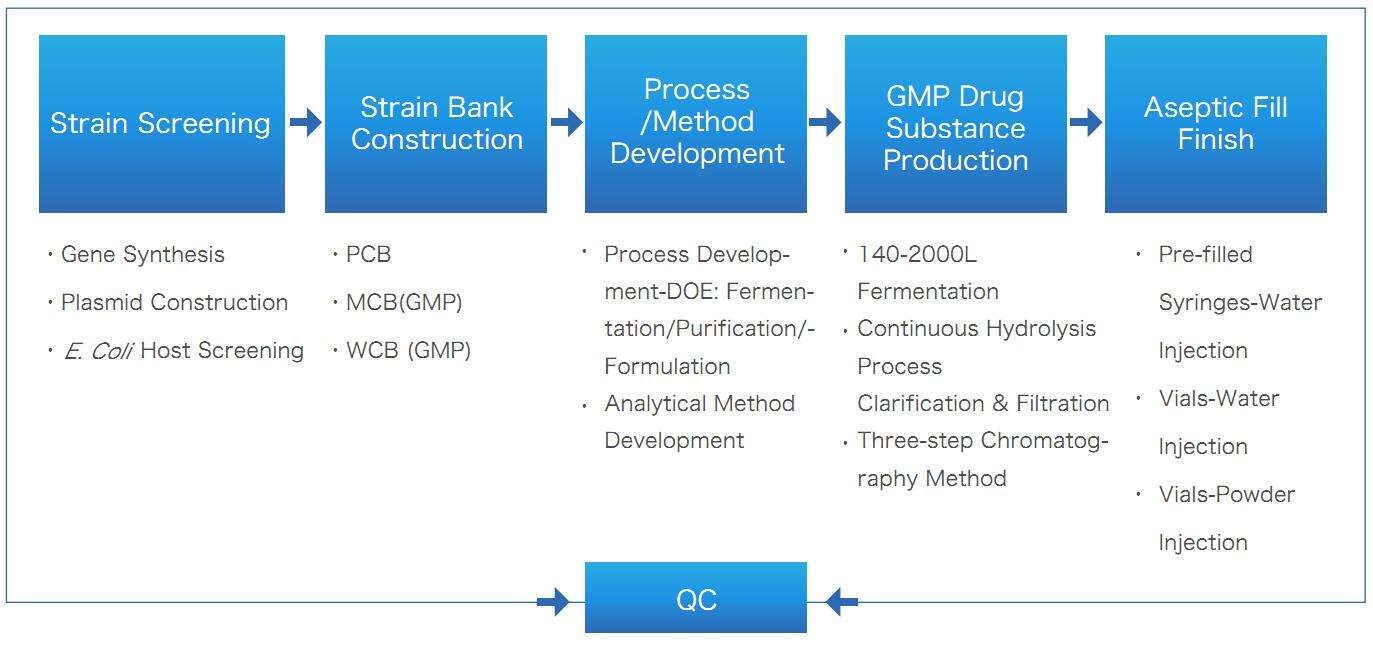
One-Stop Plasmid DNA Solution of Yaohai Bio-Pharma
Deliverables
| Grade |
Deliverables |
Specification |
Applications |
| non-GMP |
Drug substance |
0.2~10 g (plasmid DNA) |
Preclinical studies,
Analytical method development,
Pre-stability studies,
Formulation development
|
| Drug product |
| GMP, Sterile |
Drug substance |
10~100 g (plasmid DNA) |
Investigational new drug (IND),
Clinical trial authorisation (CTA),
Clinical trial supply,
Biologic license application (BLA),
Commercial supply
|
| Drug product |
20,000vials (liquid or lyophilized), pre-filledsyringes or cartridges |
Yaohais Plasmid CDMO Services, Covering the Entire Lifecycle of mRNA
Platform Features
Plasmid DNA Fermentation Technology
- With high density fermentation, the plasmid DNA yield reached 1000 mg/L under our platform process
- No animal sources, no antibiotics added or use of antibiotics that meet regulatory requirements
- Based on QbD and DoE concept, quickly identify the influencing factors to achieve process development goals
- After 2 to 3 batches of confirmation, the pilot process is amplified step by step to reduce the risk of process scale-up and process transfer.
|
Vaccine Type
|
Customers Need
|
Yaohai's Solutions
|
|
Therapeutic DNA Vaccine
|
Fermentation process development: Focus on plasmid DNA production
|
We developed animal-free media and fermentation process of the engineered Bacteria E. coli, and the fed-batch fermentation yield exceeding 1000 mg/L of plasmid DNA.
|
Plasmid DNA Purification Process
- We formulate development and production strategies based on the complexities of the project to meet the key quality attributes of the product while enhancing plasmid recovery.
- We have established a stable and scalable continuous cleavage process, as well as a three-step chromatography process that can efficiently capture supercoiled plasmids and eliminate RNA, HCP, HCD, and endotoxins.
| Vaccine Type |
Technology Difficulties |
Yaohai's Deliverables |
| Prophylactic DNA Vaccine |
Under the original process of the customer, HCD exceeded the quality standard, and the proportion of plasmid superhelix was about 80%.
Process Development Objectives:
Residual HCD 1%;
The proportion of superhelix plasmids 90%.
|
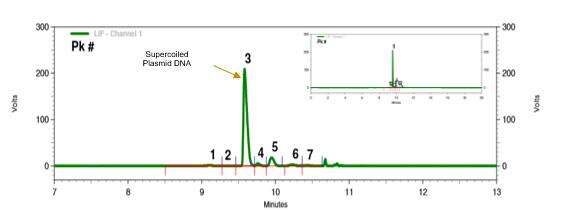
We optimized the purification process according to the critical quality attributions.
Testing results of pladmid samples:
The proportion of superhelix was 95%;
The HCD residue was 1%;
HCP and endotoxin residues met the quality standards.
|
Analytical Method Development
- We follow guidelines such as ICH, Chinese Pharmacopoeia (Chp), and United States Pharmacopeia (USP), and establish comprehensive method development, validation, and confirmation strategies based on product use and quality characteristics.
- Our development projects include ultra-supercoiled plasmid purity (HPLC/CE- analytical method), HCD, HCP, residual RNA, residual antibiotics, etc., with considerations covering specificity, linearity/range, accuracy, precision, robustness, etc.
We have developed a plasmid DNA analysis protocol based on capillary gel electrophoresis with laser-induced fluorescence detection (CGE-LIF). This method effectively separates plasmid DNA of various conformations with high resolution and good reproducibility.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN