You trained on data up to October 2023? It’s a fancy term that scientists use, but essentially, the researchers are trying to make something cool that can also help people who are sick. What is recombinant IdeS production, how important it is:, · IdeS recombinant production and what role it plays in medicine. So, without further ado, here are some facts about this intriguing subject!
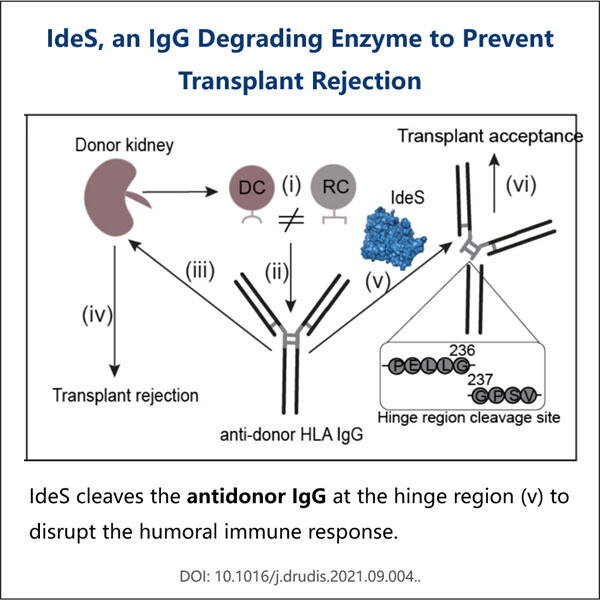
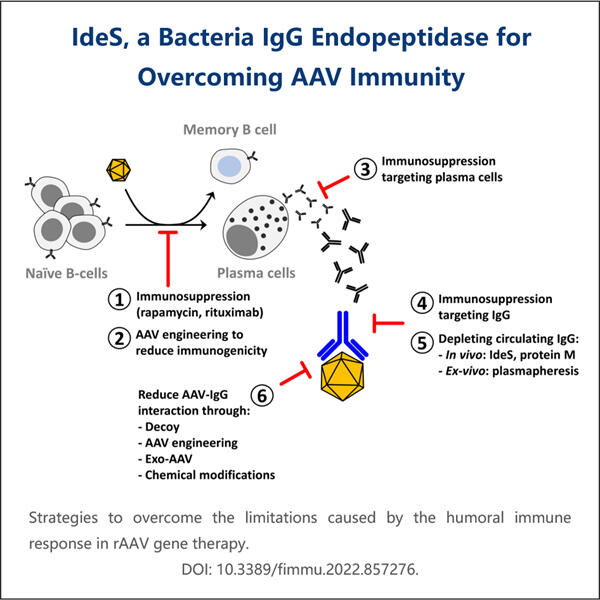
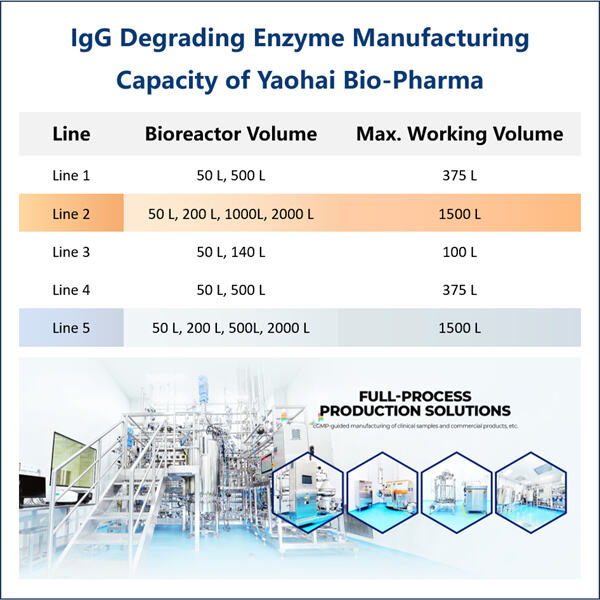
When scientists discuss "biomedical applications," they are talking about how to use medicine and technology in tandem to try to help people be healthier and happier. One rather exciting means by which they are doing that is recombinant IdeS production. The term “IdeS” is shorthand for an enzyme derived from a kind of bacteria known as Streptococcus pyogenes. This enzyme is big because it can destroy a bad protein in our bodies that can make us get sick. The scientists believe that developing recombinant IdeS will allow us to create medicines we need in order to feel well and treat diseases.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 কোন
কোন
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN