LNP Lipid Composition Identify and Quantity
The Significance of LNP Lipid Composition Analysis
Lipid nanoparticles (LNPs), comprising cationic lipid, cholesterol, phospholipid, and PEG-conjugate lipid, serve as prominent delivery systems for mRNA drug products. The stability and bilayer fluidity of LNPs depend on the properties and contents of the various lipids used in the formulation system. The composition of the lipids is very important for how well LNPs deliver drugs.
Yaohai-BioPharma provides solutions for identifying and quantifying mRNA-LNP lipid composition using the high-performance liquid chromatography with charged aerosol detection (HPLC-CAD) method.
Regulatory Requirements for Lipid Composition
According to the WHO regulatory considerations, “The final composition of the vaccine, including the lipids, should be described along with the quantity of the components in each presentation.”
Analytical Method
Analysis | Methods |
Lipid Composition | HPLC combined with Corona Charged Aerosol Detector (CAD) |
Procedure
Step 1. Lipid Sample Preparation
The choice of diluent is of paramount importance for the analysis of lipid components in mRNA-LNP drug products. The diluent should effectively dissolve each lipid component and disrupt any interactions between lipids and encapsulated mRNA. Potential diluents encompass ethanol, ethanol/ dimethyl sulfoxide (DMSO), ethanol/formamide, ethanol/triethylamine acetate, and others.
Step 2. HPLC-CAD
Reverse phase liquid chromatography (RP-HPLC) paired with a charged aerosol detector (CAD) has been incorporated into LNP lipid composition testing. Given that lipids lack chromophores and do not absorb ultraviolet (UV) radiation without derivatization, volatilization is typically challenging. CAD, being more sensitive, can discern a broader spectrum of lipid content. Previously, an ultrahigh performance liquid chromatography (UHPLC) coupled with a charged aerosol detector (CAD) method was formulated and applied in lipid analysis for small interfering RNA (siRNA) LNP studies.
Step 3. Data analysis
The quantity of each lipid within the sample involved calculations derived from the standard curve multiplied by the dilution factor. This process yielded the lipid content for each constituent within the mRNA-LNP sample.
Case of LNP Lipid Composition Analysis with HPLC-CAD
Based on HPLC-CAD, Yaohai-BioPharma has developed a robust method for the analysis of lipid components of mRNA-LNP. This method was validated to be linear, repeatable, precise, accurate, and specific for LNP formulations, which could support process development, formulation development, stability testing and mRNA-LNP release.
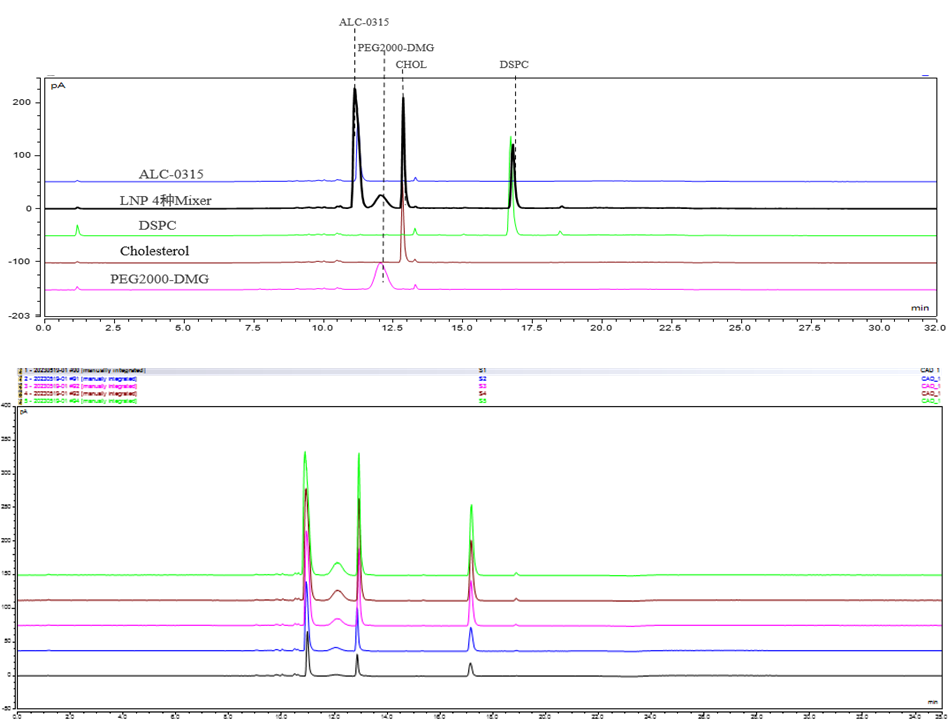
Chromatographic profile of 4 lipids by Yaohai-BioPharma’s HPLC-CAD method
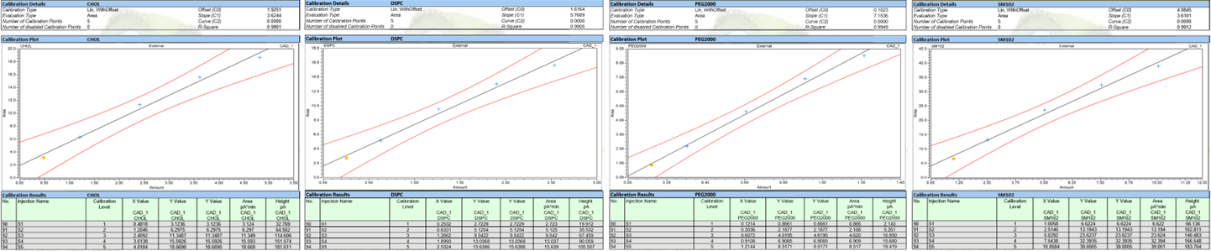
The linearity of Yaohai-BioPharma’s HPLC-CAD method (R2>0.99)

 EN
EN




