In addition to recombinant subunit vaccines targeting pathogen antigens, researchers have focused on targeting proteins in tumor cells or other metabolic pathway-related antigens. These antigens can stimulate the body to produce specific antibodies that kill tumor cells or block target metabolic pathways, achieving the goal of treating diseases.
Based on a comprehensive "recombinant protein service platform," Yaohai Bio-Pharma can provide customers with a one-stop solution from strain development and protein sample preparation to GMP production of recombinant protein vaccines. We can flexibly adjust the service process according to the customized needs, providing customers with high-quality recombinant protein Drug Substance (DS) or Drug Product (DP) in multigram or tens of grams, as well as process development and GMP production records, and testing reports.
One-stop Solution for Protein/Peptide Therapeutic Vaccines
The recombinant protein/peptide therapeutic vaccine services offered by Yaohai Bio-Pharma are also based on the [recombinant protein service platform]. For more details about the service, please refer to the "Recombinant Subunit Vaccine CDMO Services".
-
Microbial Strain Engineering and Screening
-
Microbial Cell Banking (PCB/MCB/WCB)
-
Upstream Process Development
-
Downstream Process Development
-
Formulation Development
-
GMP Manufacturing
-
Fill and Finish
-
Analytical and Testing
-
Regulatory Affairs
|
Grade
|
Deliverables
|
Specifications
|
Sample Applications
|
|
Non-GMP
|
Drug Substance
|
0.2~10 g
|
Preclinical studies,
Analytical method development,
Pre-stability studies,
Formulation development
|
|
Drug Product
|
|
GMP, Sterile
|
Drug Substance
|
2~100 g
|
Investigational new drug (IND),
clinical trial authorisation (CTA),
Clinical trial supply,
Commercial supply
|
|
Drug Product
|
20,000 vials (liquid or lyophilized), pre-filled syringes or cartridges
|
Case Study
|
Types
|
R&D Phase
|
Customer’s Need
|
Deliverables
|
|
Recombinant protein therapeutic vaccine
|
pre-IND
|
Control technology transfer risk and obtain a stable production process of drug substance;Deliver multigram recombinant protein drug substance;Ensure production activities comply with all GMP specifications.
|
• Delivery of recombinant protein drug substance that meets quality standards.
• Delivery of drug substance COA, process specifications, quality standards, production records, and other documents that fully comply with the GMP
|
|
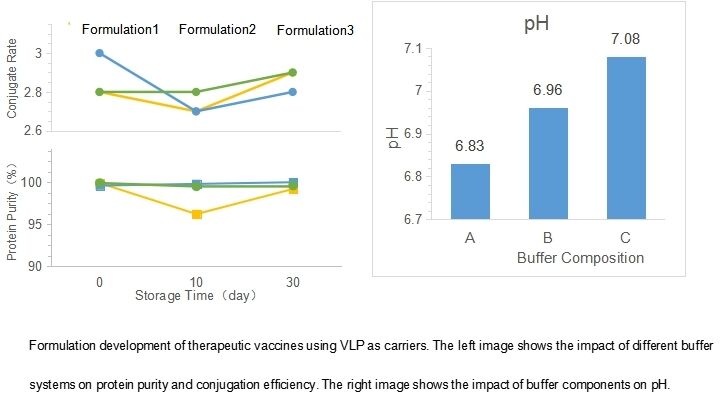
Therapeutic vaccine with VLP as carrier
|
pre-IND
|
Drug substance: Coupling of antigen-VLP carrier protein is performed in GMP workshop.Drug product: Formulation development and sterile filling.
|
• Delivery of stable drug substance formulation and drug product formulation (including adjuvants) and scalable drug product process.
• Coupling production is in progress
|
|
Note: Yaohai also provides one-stop solutions for VLP carriers, see [Carrier Protein CDMO Service]
|

 EN
EN



