Vaccinations are one of the most important preventive tools against infectious diseases. The efficacy of a vaccine depends not only on the antigen components, but also on adjuvants that are often used to stimulate the immune system more effectively. Adjuvants have several benefits, such as the reduction in the antigen amount per vaccine dose and the number of vaccination sessions, and in certain cases, they increase the stability of the antigen component, extending its half-life and indirectly improving its immunogenic power.
Many different types of adjuvants are now approved for use in vaccine manufacturing, e.g., Mineral Salts (Aluminum), Emulsions (MF59, AS03), Natural product (MPL, QS-21, Squalene), Combined adjuvants (AS01, AS02), Cytokines (Interleukin, Interferons, GM-CSF).
Timeline of vaccine adjuvant development
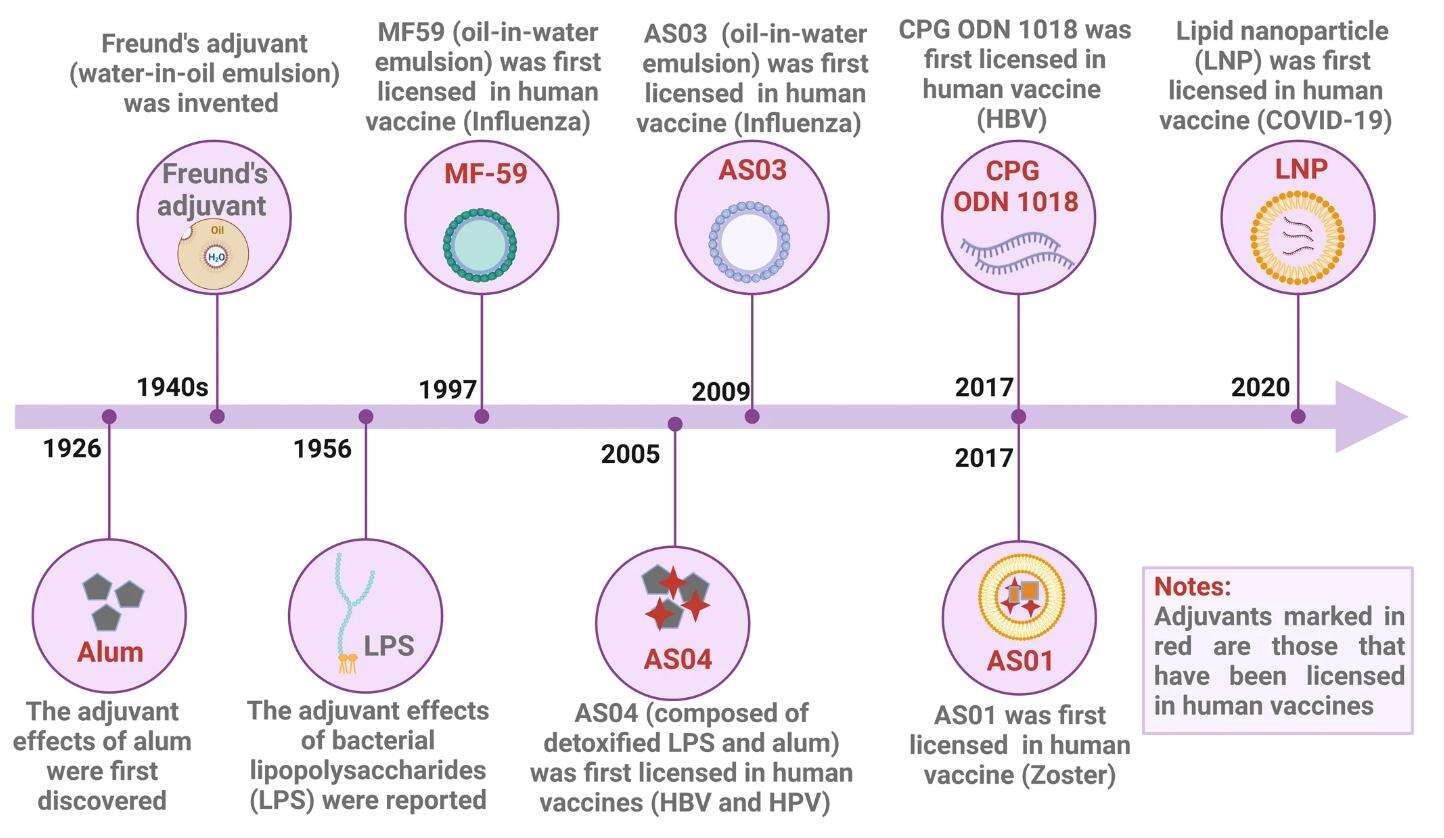
Timeline of vaccine adjuvants development. (Zhao T, et al. Signal Transduct Target Ther. 2023 Jul 19;8(1):283.)
Some of Licensed Adjuvanted Vaccines for Human Use
| Trade Name |
Type |
Adjuvant |
| CERVARIX |
Human Papillomavirus vaccine (types 16, 18) (recombinant) |
AS04 containing 3-O-desacyl-4’-monophosphoryl lipid A (MPL) adsorbed on Aluminum hydroxide |
| FENDRIX |
Hepatitis B vaccine (recombinant) |
AS04 containing MPL adsorbed on Aluminum hydroxide |
| FLUAD |
Inactivated influenza vaccine,surface antigen |
MF59, squalene-based adjuvant |
| NUVAXOVID |
COVID-19 Vaccine (recombinant) |
Matrix-M containing Fraction-A and Fraction-C of Quillaja saponaria Molina extract |
| SHINGRIX |
Herpes zoster vaccine (recombinant) |
AS01B containing Quillaja saponaria Molina plant extract, fraction 21 (QS-21) |
| MOSQUIRIX |
Plasmodium falciparum and hepatitis B vaccine (recombinant) |
AS01E containing QS-21 and MPL |
Yaohai’s Adjuvants Manufacturing Capabilities
Under our GMP workshop with biosafety levels BSL-1 and BSL-2, Yaohai Bio-Pharma provides Contract Manufacturing Services for microbial or plant-derived adjuvants, as well as recombinant cytokines as adjuvants.
More specifically, we offer customized solutions for GMP-grade MPL, QS-21, Recombinant Cytokines, and other vaccine adjuvants.
Equipment
For microbial-derived adjuvants (like MPL), or recombinant cytokines, you can choose large-scale Stainless Steel Fermenters varying in volumes of thousands of liters, matched with Centrifugal, Hollow fiber and low-to-high pressure Chromatography Systems


Fermentation system 2000 L Disc stack centrifuge


High-potency Manufacturing Suite High-pressure chromatography

 EN
EN







