The Significance of Formulation Development
Biological drugs, such as recombinant proteins or peptides, are less stable than small molecule drugs. If a drug cannot be delivered in a stable form, it may not even go beyond first-in-human (FIH) studies.
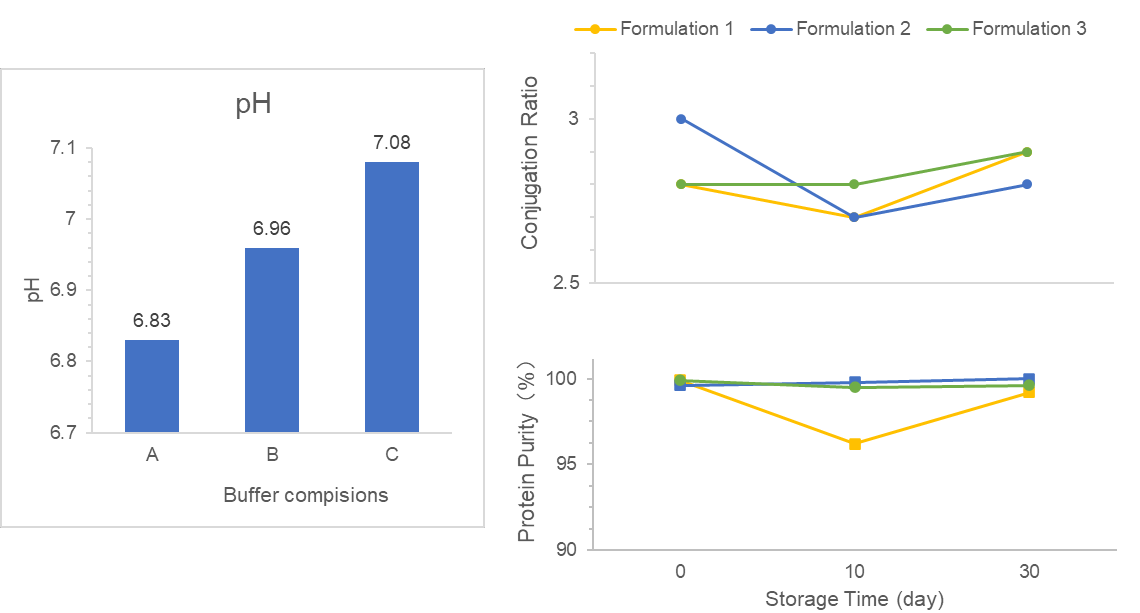
Therefore, formulation development is one of the most critical aspects in the biological lifecycle for ensuring drug quality, efficiency, and stability during manufacturing, transport, long-term storage, and administration.
Keywords: Biopharmaceutical formulation development and optimization, biologics dosage form, drug formulation composition, pre-formulation studies, formulation research, formulation screening
Application: Biopharmaceutical industry, human medicine, animal medicine, vaccine, recombinant large molecule biologics, biologics, biological reagent
Formulation Development Services of Yaohai Bio-Pharma
Liquid and lyophilized (freeze-dried) formulations currently represent the most common administration routes for biologics. Yaohai Bio-Pharma specializes in developing liquid Drug Substance (DS) or Drug Product (DP) as well as lyophilized DP in vial or prefilled syringes for different administration routes, including intravenous (IV), subcutaneous (SC), intravitreal (IVT), and inhalation (INH).

 EN
EN