Pathogenic bacteria such as Haemophilus influenzae type B, meningococcal, pneumococcal, and typhoid Salmonella have a capsular structure, which can cause invasive infections in children. Capsular polysaccharides are important factors causing these bacterial infections and are the target antigens for vaccine development.
Vaccines based on bacterial polysaccharides include polysaccharide vaccines and conjugate vaccines.
Polysaccharide vaccines use polysaccharide antigens as active ingredients.
Conjugate vaccines are formed by coupling polysaccharides with a carrier protein such as toxoid and virus-like particle (VLP), which can enhance the protective effect of the vaccine.
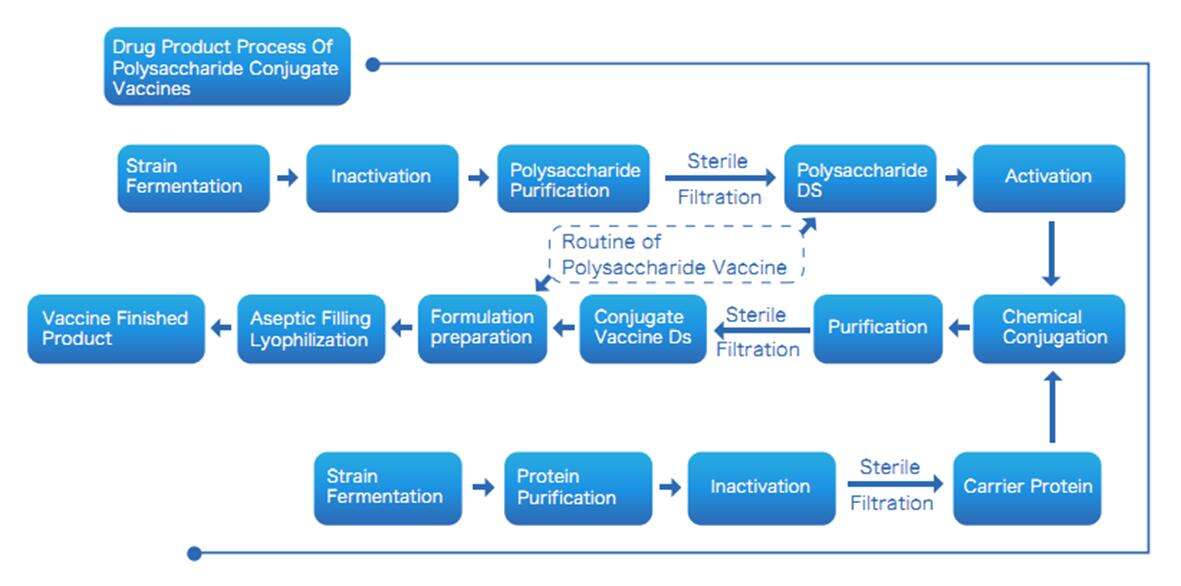
Yaohai Bio-Pharma has more than a decade of microbial CDMO experience. Based on the GMP workshop with Biosafety Level 1 (BSL-1) and Biosafety Level 2 (BSL-2), we provide a one-stop solution for microbial strain development, fermentation, extraction and purification of polysaccharides and carrier proteins, conjugation, and aseptic filling.
According to the customized needs of customers, we provide customers with intermediates, vaccine drug substance (DS, API) or drug produce (DP) that meet quality standards, as well as GMP production records and test reports.
Drug Product Process of Polysaccharide Conjugate Vaccines
Deliverable
|
Grade
|
Deliverables
|
Specification
|
Applications
|
|
GMP, BSL-1/BSL-2
|
Intermediate substance
|
Polysaccharide antigen
|
Investigational new drug (IND),
Clinical trial authorisation (CTA),
Clinical trial supply,
Biologic license application (BLA),
Commercial supply
|
|
Carrier protein
|
|
Drug substance
|
Conjugate vaccine
|
|
Drug product
|
Vials (liquid)
|
|
Vials (lyophilized)
|
|
Other Dosage Forms
|

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN